Archive for June 2019
Medtronic Recalls Some Insulin Pumps as FDA Warns They can be Hacked
The U.S. Food and Drug Administration is warning patients and health care providers that certain Medtronic MiniMed insulin pumps are being recalled due to potential cybersecurity risks and recommends that patients using these models switch their insulin pump to models that are better equipped to protect against these potential risks. To date, the FDA is not…
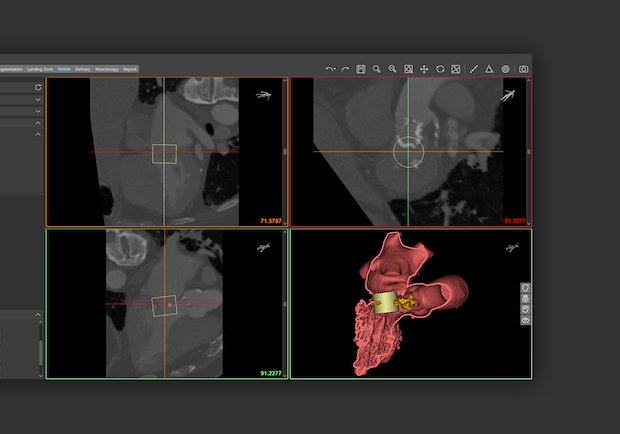
Read MoreMaterialise Receives FDA Clearance for Cardiovascular Planning Software Suite
Materialise, a global 3D printing software and solutions company, has received FDA clearance for its Mimics Enlight cardiovascular planning software suite. The first release will support clinicians planning complex transcatheter mitral valve replacement (TMVR) procedures. Mimics Enlight is based on the strengths of Materialise’s Mimics Innovation Suite, which has helped clinicians produce patient-specific 3D models…
Read MoreRa Medical Launches Large Registry Trial for Peripheral Laser Device
Ra Medical said today that it launched a large registry study to follow patients treated with its laser device for restenosis in the peripheral arteries. Carldbad, Calif.-based Ra Medical’s Dabra device uses laser radiation ablation to bore through blockages in affected arteries. It’s designed to minimize damage to the surrounding vessel tissue. The 2,500-patient Results trial…
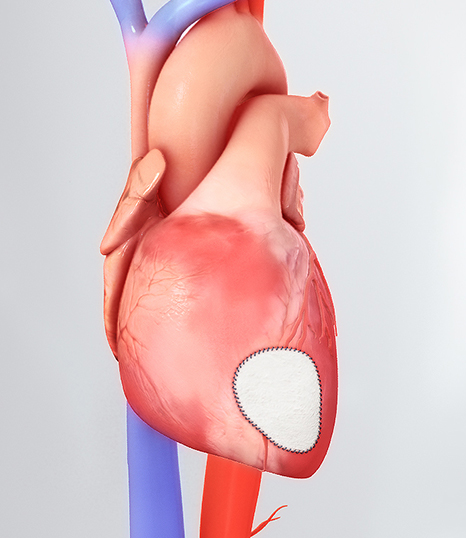
Read MoreCorMatrix Cor PATCH for Epicardial Tissue Repair Gets FDA Clearance
CorMatrix® Cardiovascular, Inc., a leading developer of regenerative cardiovascular medical devices, today announced FDA 510(k) clearance for the Cor™ PATCH. The Cor™ PATCH is indicated for epicardial tissue support and repair in adult patients. The Cor™ PATCH epicardial patch is the first epicardial cardiovascular medical device composed of first next generation CorMatrix® ECM® to be…
Read MoreMedtronic Partnership Reveals New Details About Surgical Robotics Project
Medtronic won’t unveil its new robot-assisted surgery platform until the fall, but a recent announcement from the company offered a sneak peek at what’s coming. The medical device giant yesterday disclosed a partnership with Karl Storz SE & Co. (Tuttlingen, Germany) to use Karl Storz’s three-dimensional vision systems and visualization components into Medtronic’s upcoming robot-assisted surgical…
Read MoreZoll Buys AED Developer Cardiac Science
Cardiac Science Corporation, a leading provider of automated external defibrillators (AEDs), related services and accessories, today announced it has entered into a definitive agreement to be acquired by ZOLL® Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions. CSC is a portfolio company of Aurora Resurgence, a Los Angeles-based…
Read MoreNexus Ultrasonic Surgical Platform from Misonix FDA Cleared
Misonix, Inc., a provider of minimally invasive therapeutic ultrasonic medical devices that enhance clinical outcomes, today announced that it received 510(k) clearance by the U.S. Food and Drug Administration (FDA) for Nexus, its revolutionary ultrasonic surgical platform. Misonix will commence the commercialization of the Nexus platform in the United States in July. Nexus is a…
Read More