Archive for July 2023
Axial3D Receives FDA Clearance for Axial3D INSIGHT™ Medical Image Segmentation Platform
Axial3D, a leader in medical segmentation and 3D solutions, today announced that it is the first to receive FDA clearance for an automated, AI-driven, cloud-based segmentation platform for orthopedic trauma, orthopedic, maxillofacial, and cardiovascular applications. This achievement is the second FDA clearance Axial3D has received for its segmentation platform, INSIGHT™, and a significant milestone for…
Read MoreUnraveling the Distinctions: Contingent vs. Retained Search Firms
In the dynamic landscape of talent acquisition, there are two prominent search firm structures: contingent and retained search firms. Understanding their divergent approaches is crucial for making the right decision in selecting your search firm. In this article, we’ll explore the nuanced differences between these two models, examining the benefits and disadvantages of each. Contingent…
Read MoreRIVANNA receives $30.5 million from BARDA to advance the Accuro XV musculoskeletal imaging system
RIVANNA®, developers of imaging-based medical solutions, announced that they have received funding totaling $30.5 million over 39 months from early execution of an option by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS). The funding will support…
Read MoreFDA Grants Clearance for UltraSight’s AI-Powered Cardiac Ultrasound Technology
UltraSight, a digital health pioneer transforming cardiac imaging through the power of artificial intelligence, announced that it has been granted FDA clearance for its AI-powered ultrasound guidance technology. The UltraSight real-time AI guidance software can assist medical professionals without sonography experience in acquiring cardiac ultrasound images at the point of care in multiple settings, allowing for…
Read MoreIotamotion Completes $12m Series A Raise to Accelerate Commercial Expansion of IotaSOFT® Insertion System
iotaMotion, Inc., a leader in developing advanced robotic-assisted systems for cochlear implant surgery, announced today the company has oversubscribed its $12 million Series A capital raise. The company closed the round with support from a select group of venture capital firms, private investors, and current shareholders. The lead investor was Research Corporation Technologies, Inc. (RCT) with participation…
Read MoreFDA Clears Numares Health Cardiovascular Diagnostic Test and Core Technology Platform
The US Food and Drug Administration has cleared a Numares Health test, the AXINON® LDL-p Test System, as a new tool physicians can use to measure lipoproteins for patients at risk for cardiovascular disease. Currently, Numares is the only company in the US selling an FDA-cleared NMR test. The FDA clearance also includes the company’s…
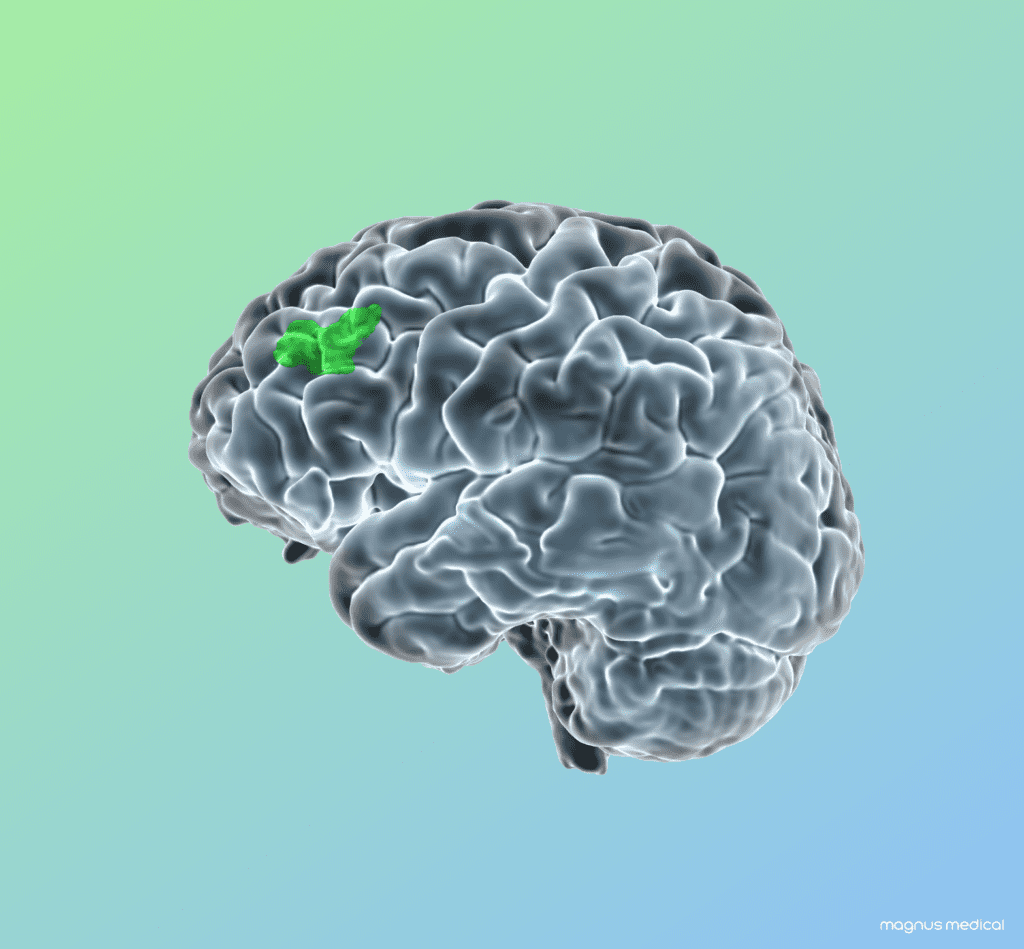
Read MoreMagnus Medical Announces First Participants Treated in Study Using SAINT Neuromodulation System for Major Depression
Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for the treatment of neuropsychiatric disorders, today announced that the first participants have been treated in the Open Label Optimization (OLO) Clinical Trial evaluating the effectiveness of the Magnus Neuromodulation System with SAINT™ Technology for the treatment of Major Depressive Disorder (MDD).…
Read MoreUroMems Announces First-Ever Smart Artificial Urinary Sphincter Implant in a Female Patient
UroMems, a global company developing innovative, mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully completed the first-ever implant of the UroActive™ smart, automated artificial urinary sphincter (AUS) in a female patient. This milestone indicates a new era for millions of women suffering from SUI, and results of this clinical study will…
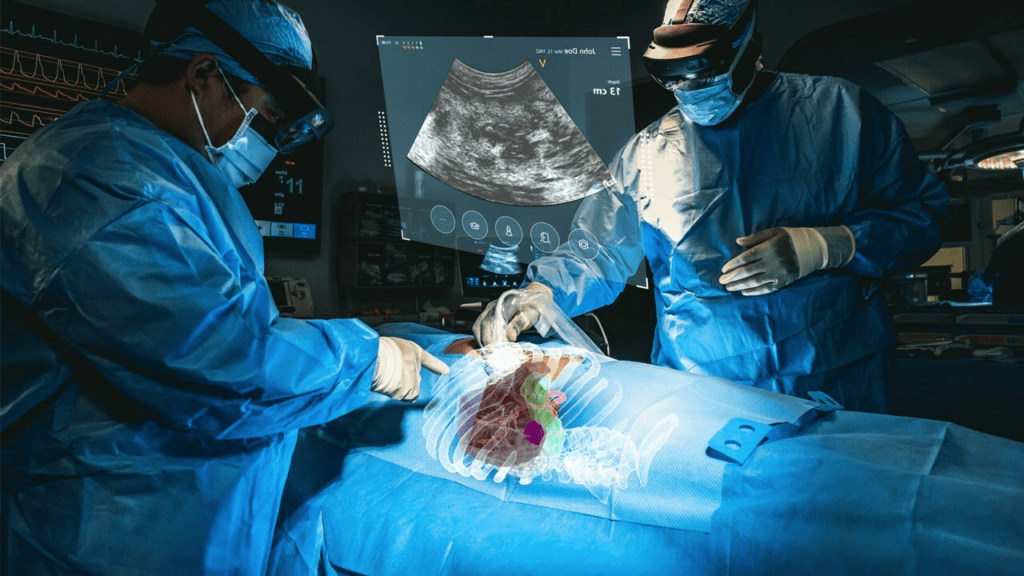
Read MoreMediView Receives FDA 510(k) Clearance for XR90 Augmented Reality-Based Visualization and Navigation Platform
MediView XR, Inc., a leading clinical augmented reality med-tech company, announced today it received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for its XR90 augmented reality-based surgical visualization and navigation platform. XR90 is intended to be used adjunctively for minimally invasive ultrasound and CT guided needle-based procedures for soft tissue and bone.…
Read MoreFrancis Medical Announces First Patient Treated in VAPOR 2 Pivotal Study for Water Vapor Ablation of Prostate Cancer
Francis Medical, Inc., a privately held medical device company developing an innovative and proprietary water vapor ablation therapy for the treatment of prostate, kidney, and bladder cancer, today announced the first patient has been treated in the company’s VAPOR 2 pivotal clinical study evaluating the safety and efficacy of its Vanquish minimally invasive water vapor…
Read More