Archive for August 2018
Stryker to acquire K2M for $1.4B
Stryker said today it inked a definitive agreement to acquire K2M for approximately $1.4 billion. In the deal, Kalamazoo, Mich.-based Stryker said it will pay $27.50 per share for each outstanding share of K2M, for a total of approximately $1.4 billion. The purchase price represents a 27% premium over K2M’s average closing price during the 90 days of…
Read MoreWright Medical to Buy Orthopaedic Company Cartiva for $435M
Wright Medical Group announced it has entered into a definitive agreement to acquire Cartiva, Inc., a private orthopaedic medical device company focused on treatment of osteoarthritis of the great toe. The transaction will add a differentiated PMA-approved technology for a high-volume foot and ankle procedure and further accelerates growth opportunities in Wright’s global Extremities business. Under…
Read MoreFDA Warning Letter Issued to Zimmer Biomet’s Indiana Manufacturing Site
Zimmer Biomet received a warning letter from the FDA about quality violations found at a plant in Warsaw, Indiana. The agency had flagged the issues after a 2016 inspection of the site, but found in a re-inspection earlier this year that the company’s resolution was not up to par. The FDA inspected Zimmer Biomet’s North…
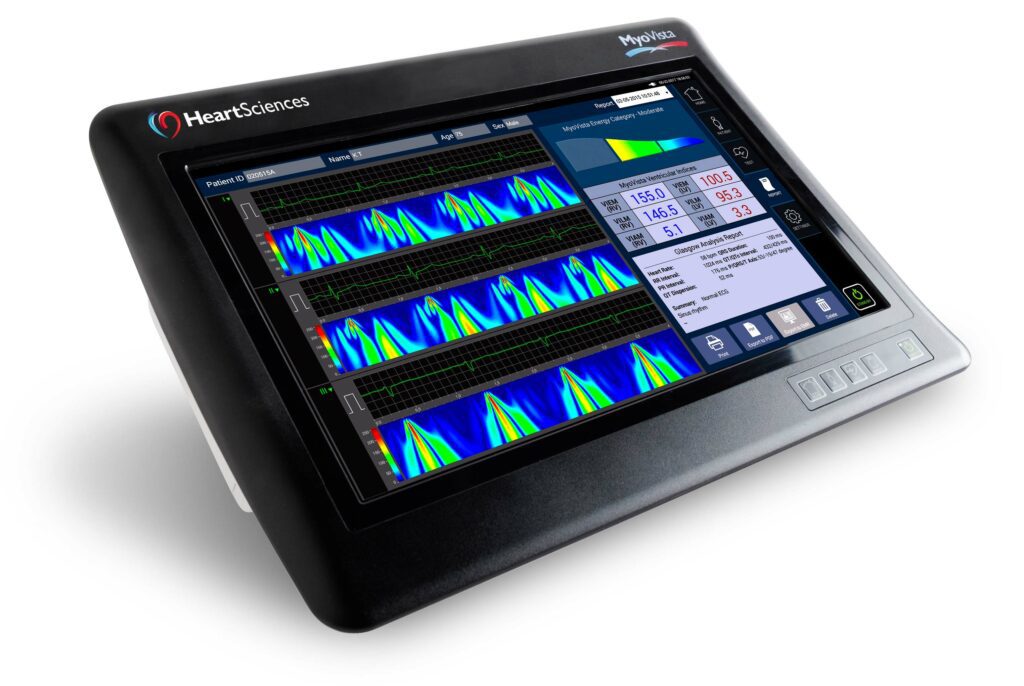
Read MoreRevolution in Electrocardiography Long in Coming, but MyoVista Fills the Bill
“Revolutionary!” and “Transformative!” are tossed around like so much jetsam in today’s spotlight-driven culture, and the medical field is not immune. But we believe a product that harvests multiple indicators of heart disease from a simple, inexpensive, first-line test has earned these superlatives. MyoVista wavECG by HeartSciences, which we saw introduced at this year’s ACC.18…
Read MoreFDA Approval for Ceterix Orthopaedics New Suture Cartridge
Ceterix® Orthopaedics, Inc., a leader in the development of cutting-edge surgical tools for orthopaedic surgeons, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of an added feature to the NovoStitch® Pro Meniscal Repair System – a size 0 suture cartridge – offering surgeons more options to repair meniscal tears. The NovoStitch Pro system enables…
Read MoreFDA grants Breakthrough Device status for Dthera’s Alzheimer’s Treatment
Dthera™ Sciences, the leading digital therapeutic company focusing on the elderly and individuals with neurodegenerative diseases, announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to the Company’s development-stage product, DTHR-ALZ. DTHR-ALZ, if granted approval, would become the first non-pharmacological prescription treatment for the symptoms of Alzheimer’s disease. The…
Read MoreVascular Changes after Smoking Hookah on par with Cigarettes
Smoking hookah for 30 minutes causes changes in arterial stiffness similar to what’s seen after someone smokes a cigarette, according to a study published in the American Journal of Cardiology. “Our findings challenge the concept that fruit-flavored hookah tobacco smoking is a healthier tobacco alternative. It is not,” lead author Mary Rezk-Hanna, PhD, an assistant professor at the…
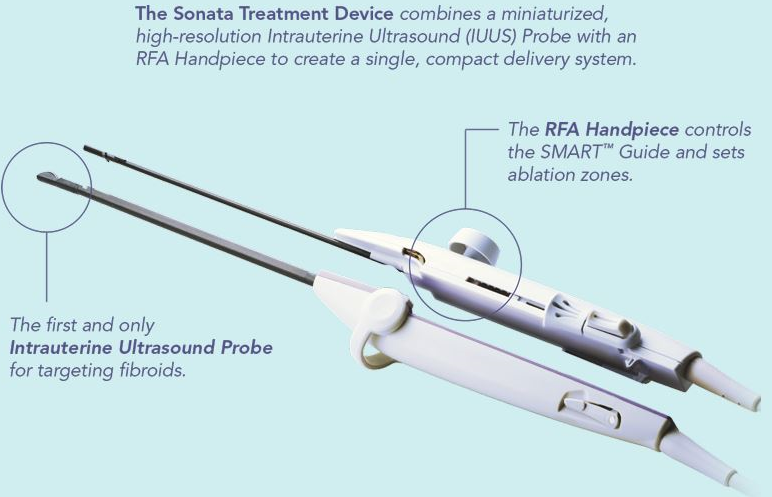
Read MoreUltrasound-Guided Transcervical Fibroid Ablation System FDA Cleared
Gynesonics, a women’s healthcare company focused on the development of minimally-invasive solutions for symptomatic uterine fibroids, today announced that it has received 510(k) clearance from the FDA to market its Sonata® Sonography-Guided Transcervical Fibroid Ablation (Sonata) System. The Sonata System combines a breakthrough integrated technology which is the first and only intrauterine ultrasound system with…
Read MoreExact Sciences Signs Partnership with Pfizer to Market Cologuard Tests
Sales representatives of Pfizer Inc., the world’s largest pharmaceutical company, will join Exact Sciences Corp.’s sales representatives in selling the company’s non-invasive screening test for colorectal cancer to physicians and health systems under an agreement announced Wednesday. Pfizer will make at least 625,000 sales calls a year for Exact Sciences under the agreement. The two…
Read MoreFDA Clears UVision360 Office Hysteroscopy Device
Luminelle DTx Hysteroscopy System has received its 510(k) clearance from the FDA with a dual-indication for both hysteroscopy and cystoscopy. The Luminelle DTx Hysteroscopy System is small, easy to use, cost effective and has the potential for an affordable, accurate patient diagnosis and/or therapeutic procedure in one doctor’s office visit. The system went from concept…
Read More