Archive for April 2020
Synaptive Medical Secures FDA Clearance for Evry
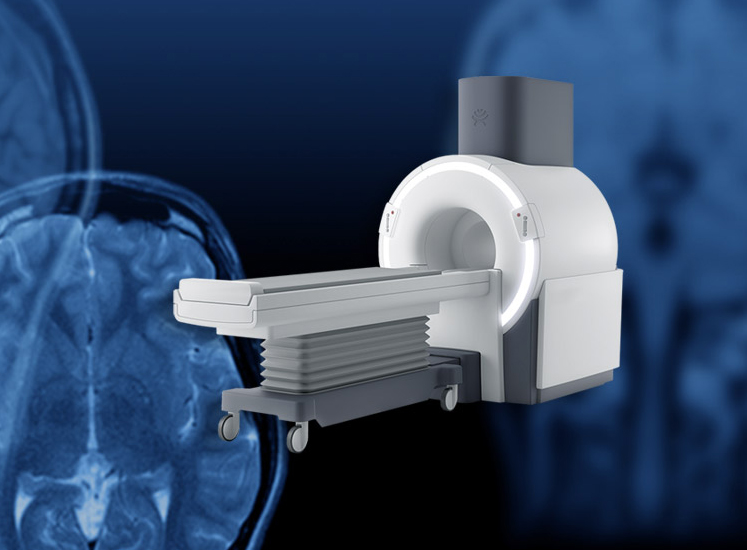
Synaptive Medical, a leader in robotic surgical visualization, announced today the company has secured clearance from the Food and Drug Administration (FDA) for its superconducting, head magnetic resonance imaging (MRI) technology, Evry™. Synaptive developed Evry to provide medical professionals with diagnostic imaging capabilities directly at the point of care in critical care settings, which was…
Read MoreAbiomed Expands Product Portfolio with Acquisition of Cardiopulmonary Support Technology to Improve Outcomes for Patients
Abiomed, maker of the Impella heart pump, has acquired Breethe, developer of a novel extracorporeal membrane oxygenation (ECMO) system that will complement and expand Abiomed’s product portfolio to more comprehensively serve the needs of patients whose lungs can no longer provide sufficient oxygenation, including patients suffering from cardiogenic shock or respiratory failure such as due to ARDS,…
Read MoreHeartVista Closes $8.65M Series A Financing Led by Khosla Ventures
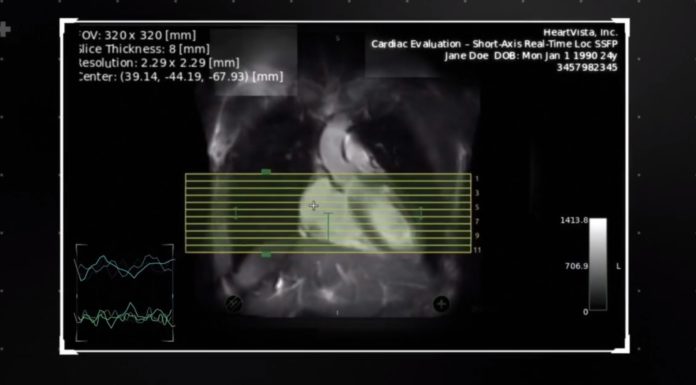
HeartVista, a pioneer in AI-assisted MRI solutions, today announced the closing of a $8.65M Series A financing round. The round was led by Khosla Ventures, Jeff Rothschild, Leslie Ventures, Open Field Capital, and additional investors. Combined with several grants from the National Institutes of Health (NIH), this latest raise brings the company’s total funding to…
Read MoreLungpacer Medical Receives FDA Emergency Use Authorization to Wean Patients Off Mechanical Ventilation During Covid Crisis
Lungpacer Medical announced today that the U.S. Food and Drug Administration has issued Emergency Use Authorization (EUA) for the Company’s novel Diaphragmatic Pacing Therapy System (DPTS) for immediate use in patients on invasive mechanical ventilators at high risk of weaning failure, including COVID-19 patients. “We are thrilled to hear about the FDA’s decision to grant…
Read MoreMolekule Launches New Commercial Product, Air Pro RX, with FDA 510(k) Class II Medical Device Clearance
Molekule, the leader in reinventing air purification, today announced that the U.S. Food and Drug Administration (FDA) cleared the 510(k) premarket notification for its new medical-grade air purifier, Air Pro RX, classifying it as a 510(k) Class II Medical Device. The Molekule Air Pro RX air purifier is intended for medical purposes to destroy bacteria and viruses in…
Read MoreCagent Vascular Announces FDA 510(k) Clearance for Balloon Catheter
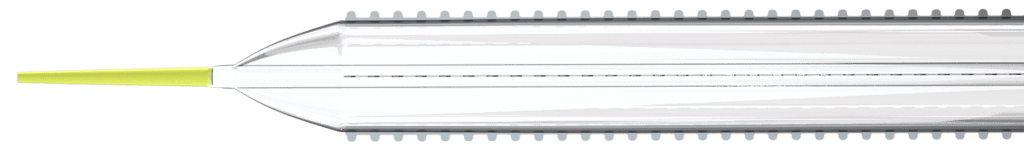
Cagent Vascular (Wayne, PA), a developer of serration technology for vessel dilatation in cardiovascular disease interventions, announces FDA 510(k) Clearance of its Serranator® PTA Serration Balloon Catheter for treating below-the-knee (BTK) lesions. The Serranator device is the first and only angioplasty balloon FDA Cleared and CE Marked that embeds serration technology into a semi-compliant balloon…
Read MoreHealthySole HOME Approved to Keep Your Home Safe From COVID-19
Researchers have proven a device using ultraviolet light technology can neutralize the COVID-19 virus and other infectious diseases on the soles of shoes by more than 99.5 percent, according to a new study. The device, called HealthySole® PLUS, is being introduced in hospitals and other settings where infection control is urgent. A home version of the…
Read MoreFDA Grants ALung Emergency Use Authorization (EUA) to the Hemolung Respiratory Assist System (RAS) for the Treatment of COVID-19
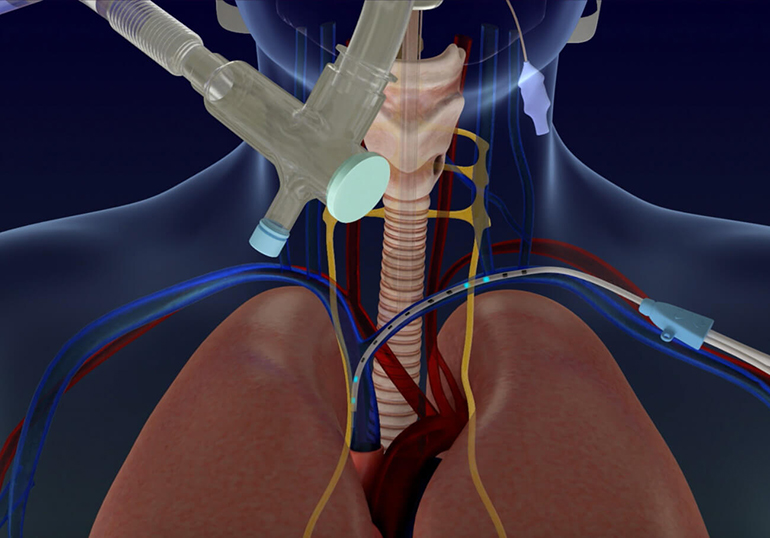
ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced that the Food and Drug Administration (FDA) has granted the Company Emergency Use Authorization (EUA) designation to the Hemolung® Respiratory Assist System (RAS) for the treatment of Coronavirus Disease 2019 (COVID-19) patients. ALung…
Read MoreFDA grants Carmell Therapeutics Expedited Review for CT-101, Bone Healing Accelerant
Carmell Therapeutics, a pioneer in the development and commercialization of innovative Plasma-based Bioactive Materials (PBMs) to accelerate bone and soft tissue healing, announced that the U.S. Food and Drug Administration (FDA) last week has granted Fast Track designation for the Company’s first product, a Bone Healing Accelerant (BHA). The Fast Track designation provides the company benefits in…
Read MoreAliveCor and OMRON Announce Global Strategic Alliance for Comprehensive Remote Cardiovascular Monitoring
AliveCor, a leader in personal ECG products, and OMRON Healthcare, Co., Ltd., a global leader in personal heart health and wellness technology, today announced a global, strategic alliance that combines AliveCor’s ECG technology with industry-leading blood pressure devices from OMRON to better serve customers and expand access to remote patient care. This partnership aligns with global…
Read More