Archive for August 2022
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System
CereVasc, Inc., a privately held, clinical-stage, medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application to initiate a pilot trial of the eShunt® System in patients who develop communicating hydrocephalus post aneurysmal subarachnoid hemorrhage (paSAH). “We are…
Read MoreMiracor Medical Announces FDA IDE Approval For PiCSO® Pivotal Study
Miracor Medical SA (Miracor Medical) has announced the approval of an Investigational Device Exemption (IDE) from the FDA, enabling the company to initiate a pivotal study with its Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) technology. The PiCSO-AMI-II multicenter, randomized trial will enroll 300 patients with anterior ST-segment Elevation Myocardial Infarction (STEMI) presenting with TIMI flow…
Read MoreMedWand Solutions Receives FDA 510k Clearance
MedWand Solutions, Inc. is pleased to announce that the company’s groundbreaking MedWand device and VirtualCare ecosystem is commercially available to enable clinical exams with its comprehensive care solutions. Poised to transform the current capabilities of telemedicine, MedWand is offering various kits including: the MedWand Evaluation Kit, the MedWand Mobile Clinic, and the MedWand Remote Clinic. By facilitating a more…
Read MoreIt’s What’s in the Middle That Counts: How to format your name on a Resume
Why you should include your Middle Initial or Name in your Resume The best-selling cookie in the world is the Oreo. In fact, if all the Oreo’s ever made were stacked, they could reach the moon and back five times and more than 450,000,000,000 have been sold since their debut in 1912. I’m one of…
Read MorePreceptis Medical Receives FDA Clearance for Expanded Labeling for Hummingbird System
Preceptis Medical, Inc., an innovative surgical technology company dedicated to providing less invasive options for pediatric patients, announced U.S. FDA clearance for expanded indications for use for the Hummingbird® Tympanostomy Tube System (TTS) for office-based pediatric ear tube procedures. Previously cleared in children 6-24 months, this new labeling allows in-office procedures in all children six…
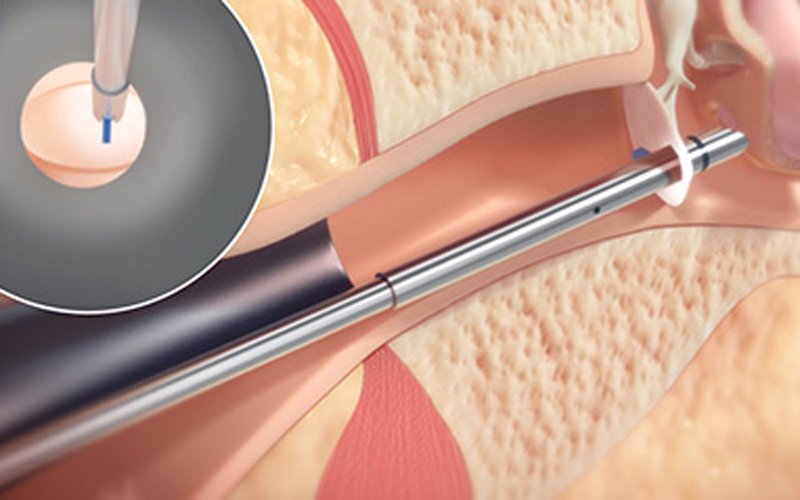
Read MoreClearPoint Neuro Announces FDA Clearance for ClearPoint Maestro™ Brain Model
ClearPoint Neuro, Inc., a global therapy-enabling platform company providing navigation and delivery to the brain, announced it has received 510(k) clearance for its ClearPoint Maestro™ Brain00 Model. Maestro is intended for automatic labeling, visualization, volumetric and shape quantification of segmentable brain structures from a set of MRI images. This software is intended to automate the…
Read MoreOSSIO Announces U.S. Launch and First Commercial Use of OSSIOfiber® Suture Anchors
OSSIO, Inc., a fast-growing orthopedic fixation technology company, announced the U.S. launch and first commercial use of OSSIOfiber® Suture Anchors, expanding patient access to the company’s growing portfolio of bio-integrative implants for use in foot/ankle, shoulder, knee, hand/wrist and elbow surgery. Gregory Berlet, M.D., founding partner at Orthopedic Foot and Ankle Center in Columbus, Ohio, recently…
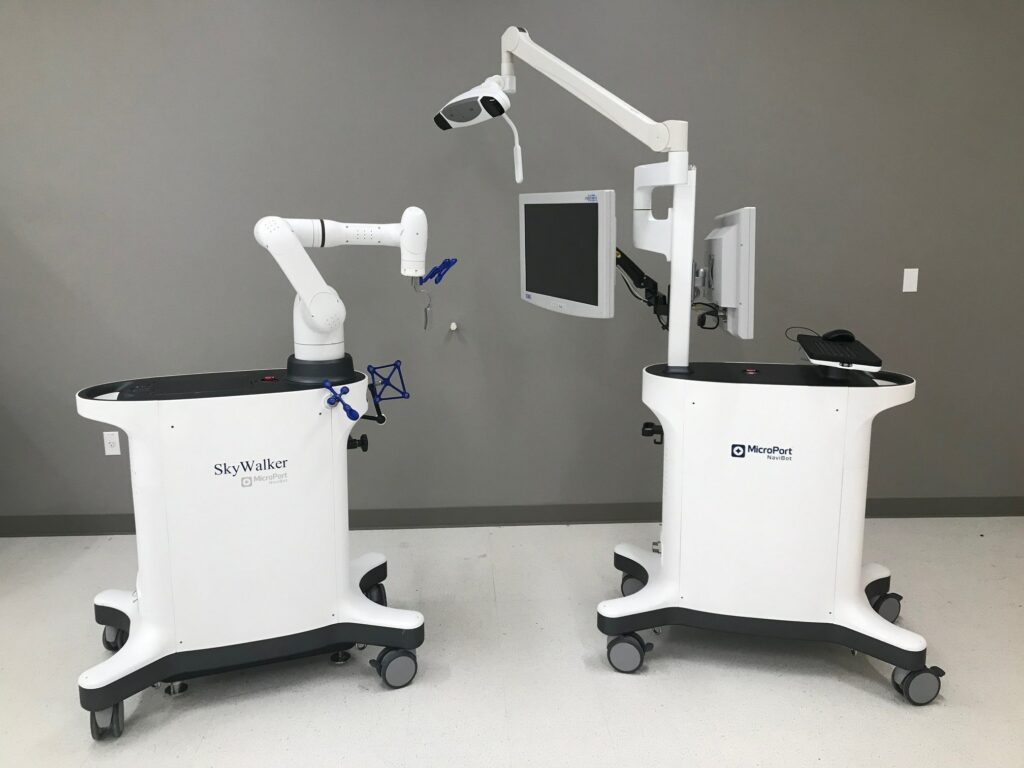
Read MoreMicroPort Navibot Receives 510(K) Clearance for its SkyWalker™ Robot-Assisted Platform for Orthopedic Applications
MicroPort Navibot has received 510(K) clearance from the Food and Drug Administration (FDA) in the United States for the SkyWalker™ System, the company’s first robot-assisted platform for orthopedic applications. The SkyWalker™ System will initially offer a robotically assisted total knee replacement solution that is compatible with the Evolution® Medial-Pivot Total Knee System. Designed based on clinical needs, MicroPort…
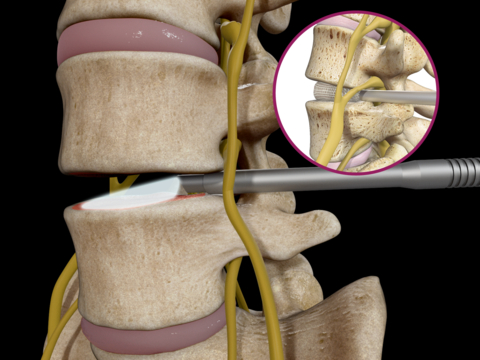
Read MoreSpineology® Launches First Fully Endoscopic, Single-Tubular Retractor Fusion System: OptiLIF® Endo
Spineology Inc., the longtime leader in ultra-minimally invasive spine surgery, announces another milestone with the limited launch of OptiLIF® Endo. This innovative, ultra-MIS system requires only one tubular retractor to seamlessly integrate endoscopes and endoscopic equipment into lumbar interbody fusion procedures. The OptiMesh® Multiplanar Expandable Implant enables this single tube system to employ the smallest diameter tubular retractor of…
Read MoreCognito Therapeutics Announces Proprietary Gamma Sensory Stimulation for 6-Months Reduces White Matter Atrophy in Alzheimer’s Disease Patients
Cognito Therapeutics, announced that its proprietary gamma sensory stimulation at 40Hz over a 6-month period reduced white matter atrophy in the brain for patients with Alzheimer’s Disease, according to new data presented at the Alzheimer’s Association International Conference 2022. Alzheimer’s Disease (AD) is the most common form of dementia. Although white matter atrophy is observed…
Read More