Archive for October 2022
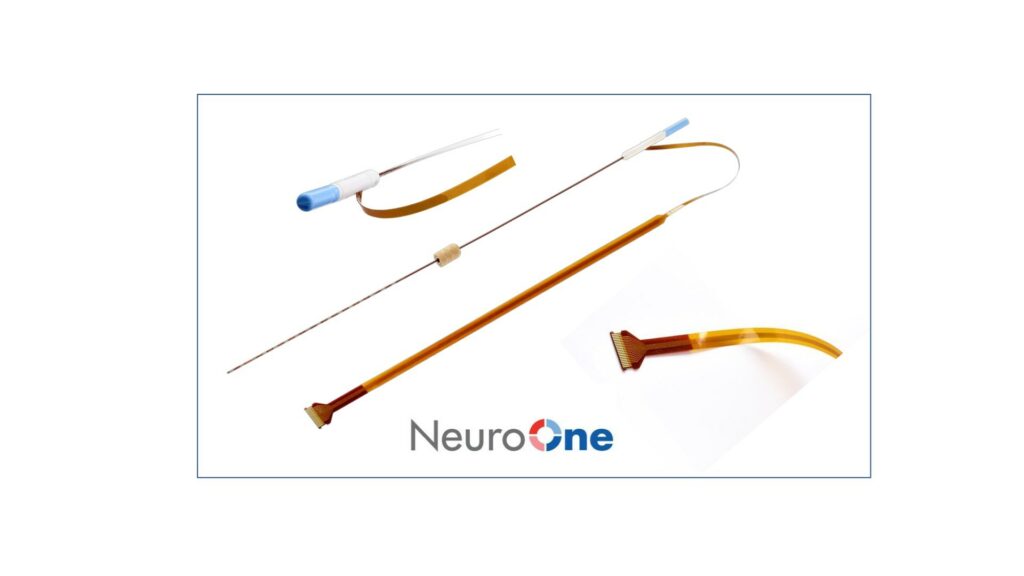
NeuroOne® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day Use
NeuroOne Medical Technologies Corporation, a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its Evo sEEG Electrode technology for temporary (less than 30 days) use with recording, monitoring, and stimulation equipment…
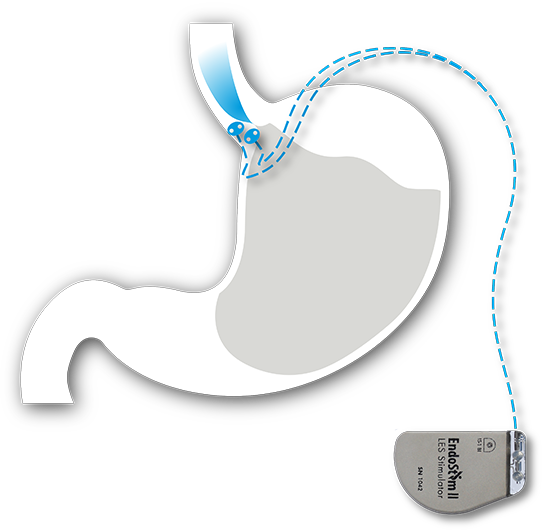
Read MoreEndoStim Receives FDA Breakthrough Device Designation for the EndoStim System for the Treatment of Drug Refractory GERD
EndoStim, a medical device company developing and commercializing a first-in-class implantable neurostimulation treatment for drug refractory gastroesophageal reflux disease (GERD), announced that the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation for the Company’s EndoStim System. FDA Breakthrough Device Designation is intended to provide patients and health care providers with timely access to…
Read MoreEyenuk secures $26 Million Series A funding to accelerate global access to AI-powered eye-screening technology
Eyenuk, Inc., a global artificial intelligence (AI) digital health company and the leader in real-world applications for AI Eye Screening™ and AI Predictive Biomarkers™, announced it has secured $26 million in a Series A financing round, bringing the Company’s total funding to over $43 million. The capital raise was led by AXA IM Alts and…
Read MoreAxoft Launches Brain Implant Technology to Treat Long-Term Neurological Disorders and is Granted FDA Breakthrough Device Designation
Axoft, a neurotechnology company, launched and announced FDA Breakthrough Device designation for its brain-machine interface (BMI) to better treat neurological disorders. The company secured $8 million in capital to fund pre-clinical studies with the FDA and to scale up prototypes of its neural implants “as soft as the brain.” The seed round investment, led by…
Read Moreulrich Medical USA™ Receives 510(k) Clearance for Flux-C™ 3D Printed Porous Titanium Cervical Interbody Prior to NASS
ulrich medical USA, Inc., a privately held medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States, announced the FDA has given 510(k) clearance of its Flux-C 3D printed porous titanium cervical interbody device. “Surgeons have many options for cervical interbodies. The Flux-C porous titanium device offers one of the…
Read MoreNevro Announces FDA Approval of HFX iQ™ Spinal Cord Stimulation System to Personalize the Treatment of Chronic Pain
Nevro Corp. (NYSE: NVRO), a global medical device company that is delivering comprehensive, life-changing solutions for the treatment of chronic pain, announced that it has received approval from the U.S. Food and Drug Administration (FDA) for the Senza HFX iQ spinal cord stimulation (SCS) system. Senza HFX iQ is the first and only Artificial Intelligence-based SCS system that…
Read MoreXACT Robotics® Receives FDA Clearance for its ACE Xtend™ Remote Control Unit
XACT Robotics®, developer of the world’s first and only comprehensive robotic system for interventional procedures, announced that its ACE Xtend™ Remote Control Unit received U.S. Food and Drug Administration (FDA) clearance, allowing users to robotically insert and steer the XACT ACE® Robotic System remotely from the control room. The first-of-its-kind feature for CT-guided percutaneous procedures…
Read MoreBody Vision Medical Accelerates International Expansion with Business Asia Consultants, Inc., Partnership
Body Vision Medical, a leader in AI-driven, intraoperative imaging, announced a new partnership with Business Asia Consultants (BAC), Inc. as part of its international expansion strategy. BAC will apply its extensive European, Asian and Latin American market development expertise to assist Body Vision Medical in realizing its market expansion goals. “We are excited about the…
Read More