Archive for October 2023
Brainomix and Nanoflex Robotics to Collaborate on an AI-Assisted Robotic System for Remote Stroke Intervention
Brainomix Ltd, an AI-powered medtech solutions company, and Nanoflex Robotics AG, a remote robotic surgical company based in Switzerland, have been awarded a grant under the “UK – Switzerland Bilateral: Collaborative R&D” program, in which the companies will work together to jointly develop an integrated remote diagnosis and treatment platform for stroke, powered by artificial intelligence.…
Read MoreFDA clears Intelivation Technologies new Hammerdesis™ Interphalangeal Fusion System
Intelivation Technologies, an innovative medical device company with a cutting-edge orthopedic portfolio announced today that it has received clearance from the Food and Drug Administration for its Hammerdesis™ Interphalangeal Fusion System. Hammerdesis™ allows surgeons to correct hammertoe deformities and degenerative issues in patients by simply affixing a distinctively designed implant on the patient’s toe joint. This one-of-a-kind…
Read MoreFDA Awards HistoSonics Clearance of its First-of-a-Kind Edison® Histotripsy System
HistoSonics®, (www.histosonics.com), the manufacturer of the Edison® System and novel histotripsy therapy platforms, announced today the marketing authorization of its “Breakthrough” platform via the U.S. Food and Drug Administration’s (FDA) De Novo Classification Request process, a rigorous pre-market review pathway for medical devices with no existing predicate. Marketing authorization makes Edison the first and only histotripsy…
Read MoreCadence Announces the Acquisition of ARC Group Worldwide’s Florida Location
Cadence, Inc., a leading provider of design, development, and contract manufacturing services to the medical device, drug delivery, and diagnostics markets, announced today that it has acquired the Florida location of ARC Group Worldwide (arcw.com), a precision manufacturer specializing in Metal Injection Molding (MIM) and cleanroom plastic injection molding. ARC Florida is located in DeLand, Fla. “Acquiring ARC Florida is…
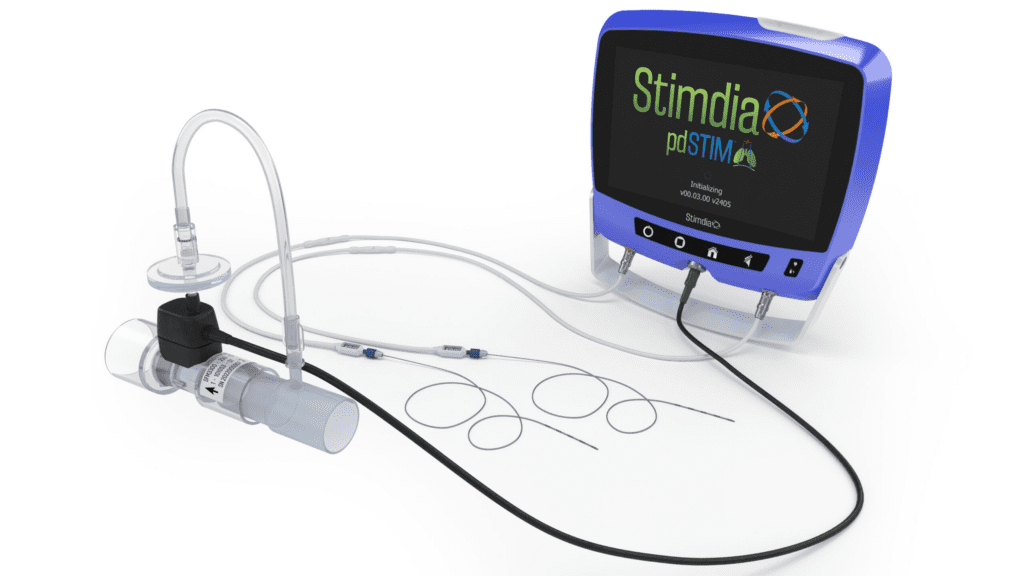
Read MoreStimdia Medical Inc. Initiates FDA Approved IDE Pivotal Trial to Investigate Impact of Neuromuscular Stimulation on Mechanically Ventilated Patients
Stimdia Medical, a medical device company developing innovative technologies to improve respiratory care, today announced the initiation of its FDA approved pivotal trial to investigate the impact of phrenic nerve stimulation (PNS) on the time to liberate patients from mechanical ventilation. The ReInvigorate Study is a randomized, controlled trial that will enroll approximately 420 patients…
Read MoreFDA Grants ExThera Multiple Breakthrough Device Designations for its Seraph® 100 Filter
ExThera Medical Corporation, a healthcare company developing and manufacturing extracorporeal pathogen technologies, announces that it has received multiple Breakthrough Device Designations from the U.S. Food and Drug Administration (FDA), for its Seraph® 100 Microbind® Affinity Blood Filter (Seraph 100). The Seraph 100 is a patented blood filter containing a biomimetic surface, similar to that found…
Read MoreEnable Injections Receives First U.S. Food and Drug Administration (FDA) Approval
Enable Injections, Inc. (“Enable”) today announced that the U.S. Food and Drug Administration (FDA) has approved the EMPAVELI Injector (enFuse®) for the subcutaneous delivery of EMPAVELI® (pegcetacoplan), which is commercialized in the United States by Apellis Pharmaceuticals, Inc. for adults with paroxysmal nocturnal hemoglobinuria (PNH). The EMPAVELI Injector is a compact, wearable injector designed to streamline the self-administration experience with minimal…
Read More