Archive for June 2018
BIOLIFE4D Successfully 3D Bioprints Human Heart Tissue
BIOLIFE4D, a biotech pioneer leveraging advances in tissue engineering to 3D print human organs viable for transplant, today announced it has successfully demonstrated its ability to 3D bioprint human cardiac tissue – specifically, a human cardiac patch. The scientific milestone was accomplished within days of the May opening of the Company’s new research facility at JLABS in…
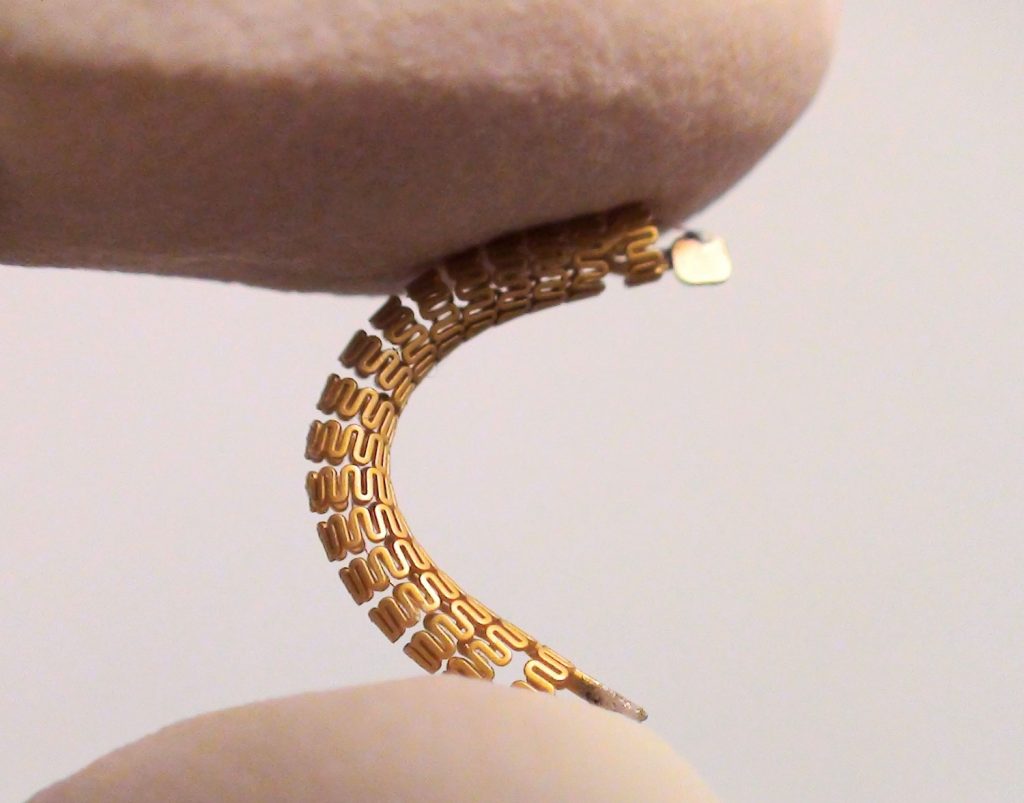
Read More“Smart Stent” Monitors Blood Flow, Detects Narrowing of Arteries
Vascular stents are some of the most commonly implanted medical devices, keeping millions of arteries open to uninterrupted blood flow. Though they tend to work well at first, too many end up being re-stenosed by new deposits of plaque and scar tissue often forms in the vicinity, blocking blood flow through the stent. Typically, renewed…
Read MoreGE to Spin Off Healthcare Division
After a year of soul searching and strategic review, GE announced its move today to spin off GE Healthcare into a standalone company over the next 12 to 18 months. The goal, the company said, is a leaner corporate structure with substantial reductions in debt. Healthcare is one of eight businesses GE operates that also…
Read MoreFDA Approves First Marijuana-derived Drug for Severe Forms of Epilepsy
The U.S. Food and Drug Administration today approved Epidiolex (cannabidiol) [CBD] oral solution for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients two years of age and older. This is the first FDA-approved drug that contains a purified drug substance derived from marijuana.…
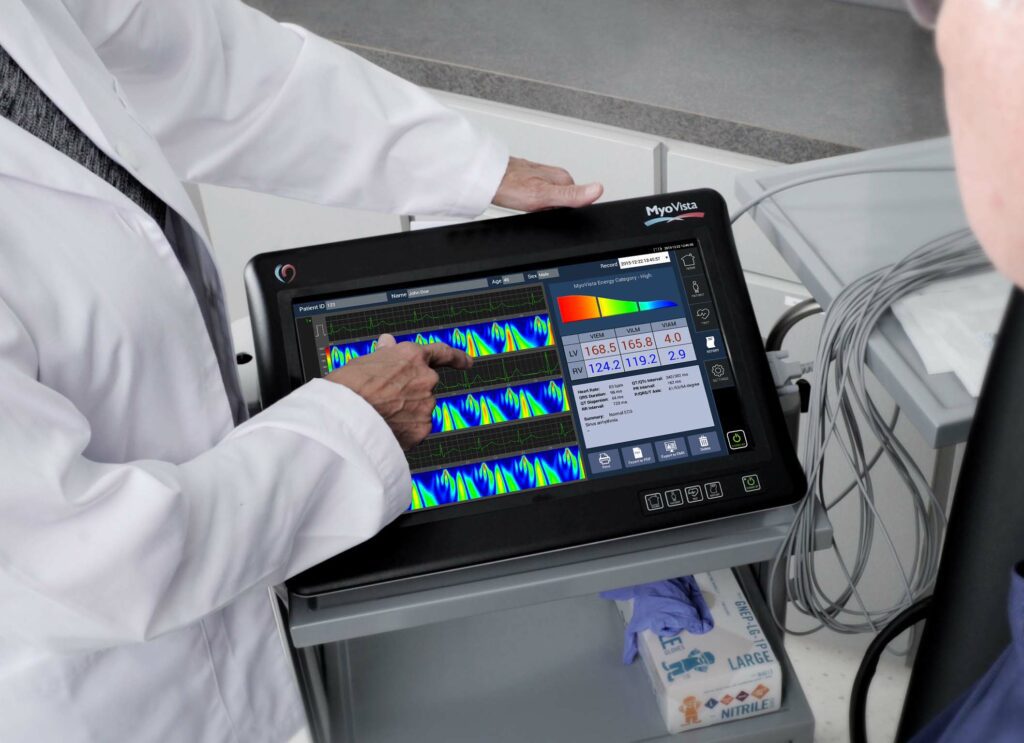
Read MoreHeartSciences Named “Start-Up to Watch”
SOUTHLAKE, Texas–(BUSINESS WIRE)–HeartSciences is delighted to have been highlighted as “Start-Up to Watch” in the June 8, 2018 issue of the MedTech Strategist. The article titled “HEARTSCIENCES: ARTIFICIAL INTELLIGENCE IMPROVES FRONTLINE RISK STRATIFICATION FOR HEART DISEASES” discusses HeartSciences’ role in addressing the current challenges and significant unmet need to identify heart disease at an early stage and reduce the…
Read MoreWhite House Proposes 30% Cut to FDA Headcount
The Trump administration this week submitted a proposal that would cut the budget and staff at the FDA, as well as reorganizing the agency to remove food regulation from its purview, according to a new Endpoints News report. The proposal would cut $1.3 billion in resources from the agency and 5,000 members of its staff and leave…
Read MoreWorld’s First Long-Term Implantable Glucose Monitoring Device Now FDA Approved
GERMANTOWN, Md., June 21, 2018 — Senseonics Holdings, Inc. (NYSE American: SENS) today announced the U.S. Food and Drug Administration has approved its Pre-market Approval (PMA) application to market the company’s Eversense® Continuous Glucose Monitoring (CGM) System to people with diabetes in the United States. The system is the first and only CGM system to feature…
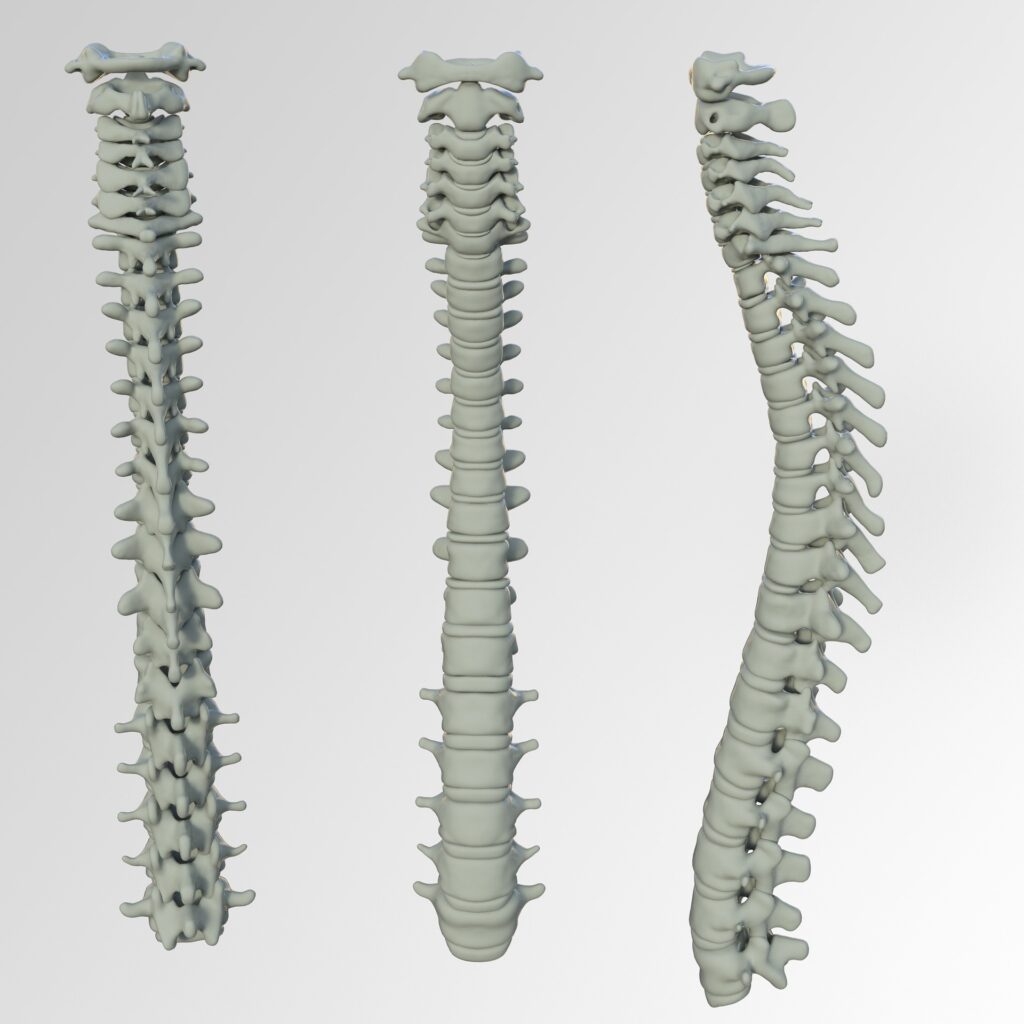
Read MoreMedtronic Receives Expanded Indication for Kyphon HV-R Bone Cement
Medtronic seems to be on a roll when it comes to innovation. The Dublin-based company has received the greenlight from FDA for an expanded indication of the Kyphon HV-R Bone Cement. Under the new indication, Medtronic can market Kyphon for the fixation of pathological fractures of the sacral vertebral body using sacral vertebroplasty or sacroplasty.…
Read MoreGE Booted From the Dow After More Than 100 Years
For the first time in 110 years, General Electric will not be a member of the elite Dow Jones Industrial Average. S&P Dow Jones Indices announced on Tuesday that the iconic maker of light bulbs and jet engines will be replaced in the 30-stock index by Walgreens Boots Alliance. GE (GE) was an original member of the…
Read MoreScammers Trick Seniors with ‘Free’ Medical Devices, Then Bill Medicare
Nancy DeLozier looked at the array of medical equipment on the floor of her living room and called it junk. The 84-year-old Northwest Side woman said she received the items in the mail but neither wants nor needs them. One shipment, from February 2017, included a back brace and knee braces. A second shipment, which…
Read More