Archive for January 2020
BIONIK Laboratories Announces Regulatory Approval and First Sale of its InMotion ARM Robotic Technology by its Exclusive Distributor in South Korea
BIONIK Laboratories Corp., a robotics company focused on providing rehabilitation and assistive technology solutions to individuals with neurological and mobility challenges from hospital to home, today announced it has received regulatory approval in South Korea, and that its exclusive distributor Curexo secured the first sale for BIONIK’s InMotion® robotic technology in the country, out of…
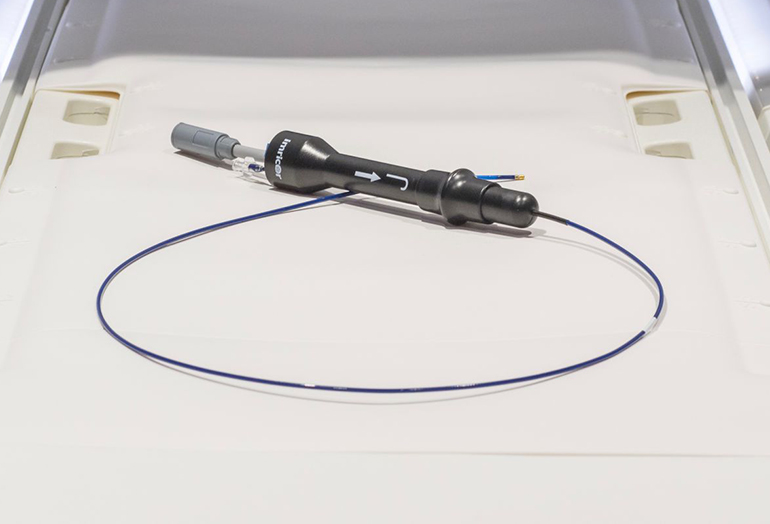
Read MoreImricor’s MRI-Compatible Ablation Catheters Cleared in Europe for Cardiac Arrhythmia Treatment
Imricor Medical Systems, Inc. (ASX:IMR) today announces that it has received CE mark approval for its Vision-MR Ablation Catheter and Vision-MR Dispersive Electrode. This follows earlier CE mark approval for Imricor to place its Advantage-MR EP Recorder/Stimulator System on the market in Europe. Imricor is the first and only company to offer cardiac ablation devices for…
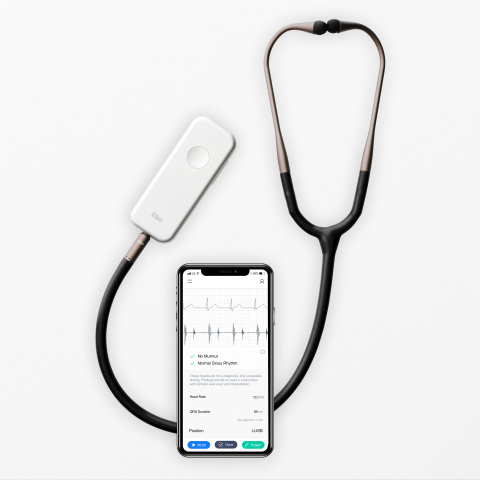
Read MoreFDA Clears Eko’s AFib and Heart Murmur Detection Algorithms, Making It the First AI-Powered Stethoscope to Screen for Serious Heart Conditions
Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, announced today that the U.S. Food and Drug Administration has cleared a suite of algorithms that, when combined with Eko’s digital stethoscopes, will enable healthcare providers in the U.S. to more accurately screen for heart conditions during routine physical exams.…
Read MoreBioIntelliSense Announces FDA Clearance of the BioSticker, a Single-Use Medical Device Enabling 30 Days of Continuous Vital Signs Monitoring
BioIntelliSense, Inc., a continuous health monitoring and clinical intelligence company, today announces the U.S. commercial launch of its medical grade Data-as-a-Service (DaaS) platform and FDA 510(k) clearance of the BioSticker™ on-body sensor for scalable remote care. BioIntelliSense offers a new standard for Remote Patient Monitoring (RPM) by combining an effortless patient experience with medical grade…
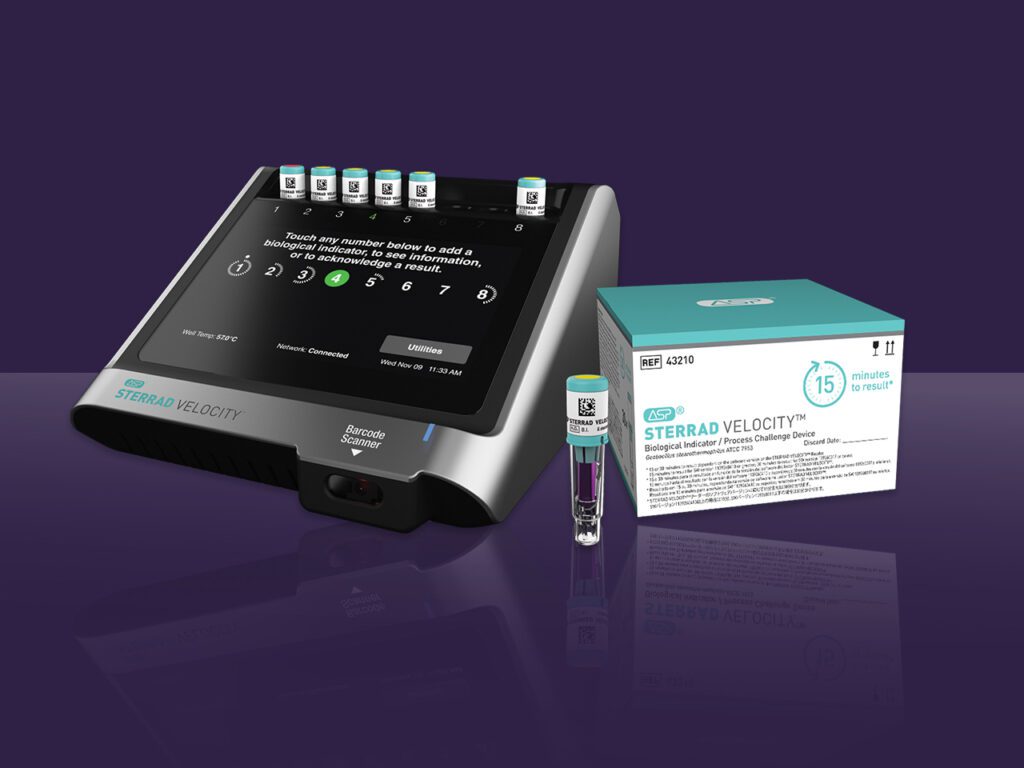
Read MoreAdvanced Sterilization Products Unveils the Fastest Biological Indicator for Hydrogen Peroxide Sterilization
Advanced Sterilization Products (ASP) announced today it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a 15-minute time to result on the STERRAD VELOCITY® Biological Indicator (BI)/Process Challenge Device (PCD) for use in STERRAD® Systems.* The 15-minute time to result is up to 38% faster than competition and offers the fastest way to…
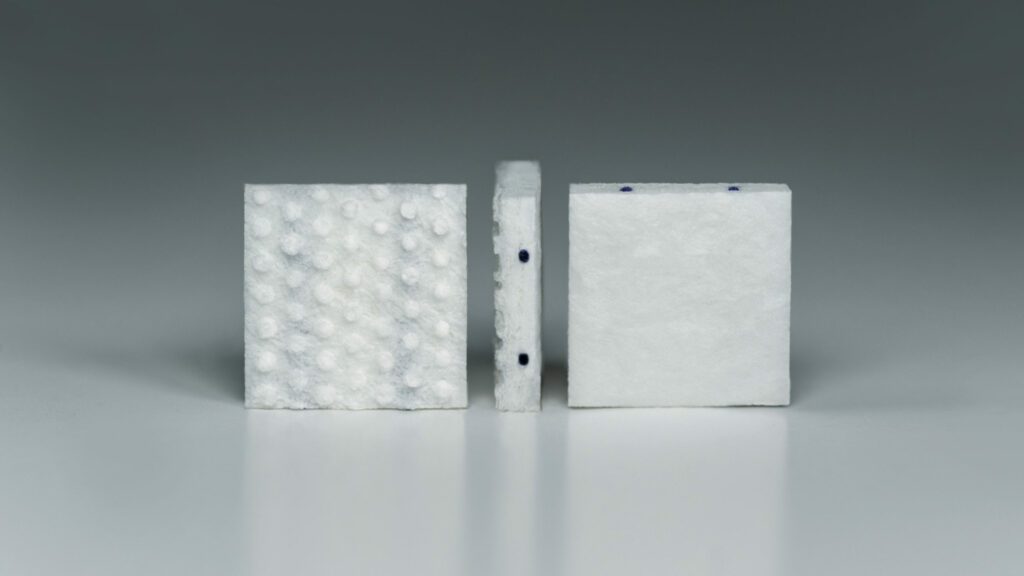
Read MoreGT Medical Technologies Announces FDA Clearance of Expanded Indication for GammaTile Therapy
GT Medical Technologies, Inc., a company dedicated to improving the lives of patients with brain tumors, today announced that the U.S. Food and Drug Administration (FDA) has cleared an expanded indication for GammaTile® Therapy. Patients with newly diagnosed malignant brain tumors are now eligible to receive the FDA-cleared surgically targeted radiation therapy (STaRT). “We are pleased…
Read MoreCardinal Begins Recall of 9.1M Surgical Gowns Amid Sterility Concerns
Details of the quality problems and Cardinal’s response to them have trickled out in January. Cardinal told customers it learned of problems with environmental conditions at a site that makes gowns last week, leading to a voluntary product hold. At that stage, Cardinal wanted customers to segregate and discontinue use of the affected surgical gowns.…
Read MoreDimer Offers New Germ-Killing Robot to Disinfect Airplanes at Key US Airports to Protect Passengers from Coronavirus Outbreak
Dimer UVC Innovations is offering its GermFalcon®, a germ-killing robot that sanitizes airplanes, to assist airlines and federal agencies disrupt the spread of a new pneumonia-like illness originating in the Wuhan region of China. The service is being offered at no expense to airlines at select U.S. airports during this crisis. The new virus is a…
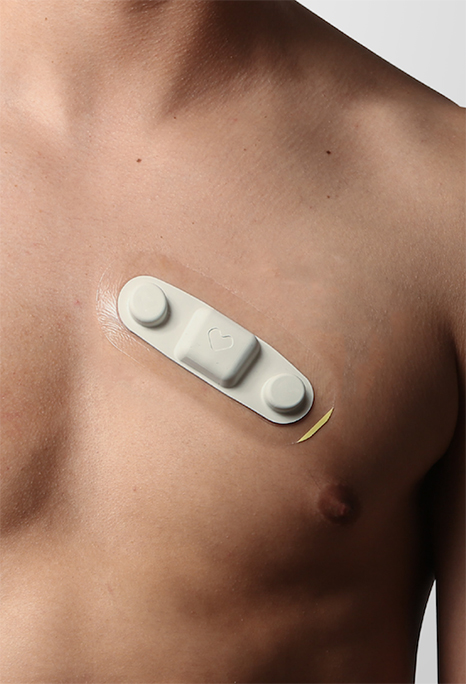
Read MoreVivaLNK Announces World’s First FDA Cleared Wearable ECG Sensor Platform
VivaLNK, a leading provider of connected healthcare solutions, today announces it received FDA clearance for its Continuous ECG Platform. Consisting of reusable wearable ECG sensors and associated software development kit (SDK), the sensor platform gives developers and providers direct control over data, and represents the first of its kind to receive FDA clearance. One of…
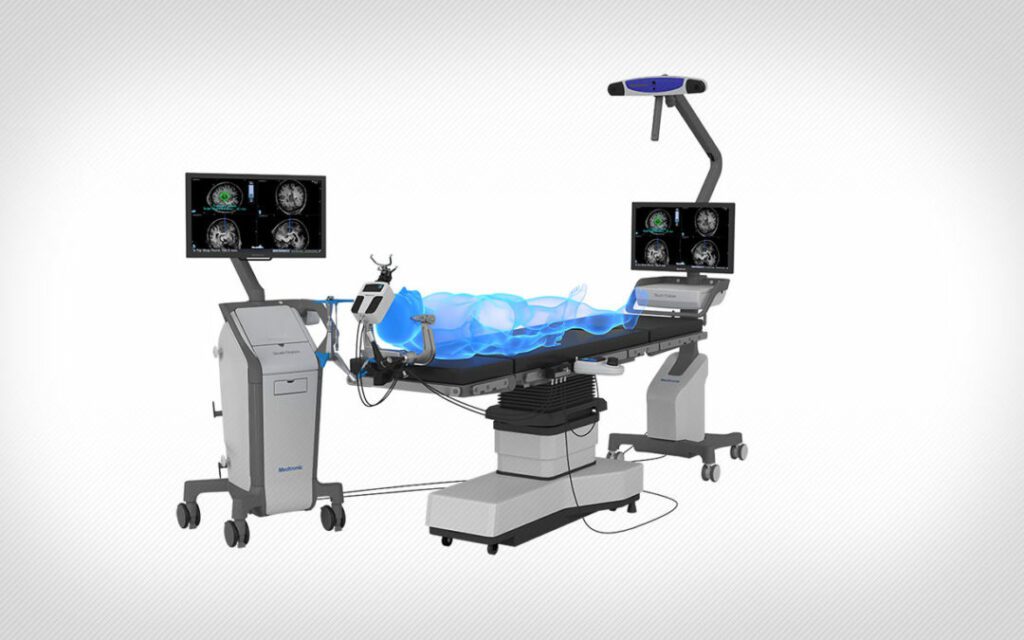
Read MorePhoenix Children’s Is the First-Ever Health System in the U.S to Use Medtronic Stealth Autoguide Cranial Robotic Guidance Platform for Neurosurgery
Nationally ranked pediatric leader, Phoenix Children’s Hospital, is the first-ever health system in the U.S. to receive and deploy the newly FDA-cleared Medtronic Stealth Autoguide™ platform. Medtronic, a global leader in medical technology, chose Barrow Neurological Institute (BNI) at Phoenix Children’s as its first partner using this robotic technology. The highly advanced surgical tool is…
Read More