Archive for August 2023
Your Recruiter (and other businesses) may not be able to text you today… Navigating New FCC Text/SMS Regulations in the US: What You Need to Know
There are some important changes in the SMS world that will affect your business/career search/online shopping habits and more. We at Legacy MedSearch believe in keeping our network in the loop, so here’s a quick summary. The What and Why Starting August 31st, the Federal Communications Commission (FCC) is rolling out stricter regulations for automated…
Read MoreBionicM Earns FDA Registration and Class II Exempt Device Listing for Bio Leg™; Its New Motor-Robotic Prosthetic Knee
BionicM, an innovative Japanese medical device startup, is pleased to announce that it has received U.S. Food and Drug Administration (FDA) 510(k) exemption registration for Bio Leg™; its robotic prosthetic knee. This registration marks a significant milestone in the company’s commitment to deliver its robotic prosthetic knee to the USA market in 2024 with Japanese-proven technologies. “Having…
Read MoreMarrow Access Technologies Partners with Spartan Medical to Provide Novel Cartilage Repair Therapy to US Veterans and Department of Defense Service Members
Marrow Access Technologies announced today that it signed a distribution agreement with Spartan Medical. The distribution agreement will provide patients in the Department of Veterans Affairs (VA) and Department of Defense (DoD) access to the SmartShot® Marrow Access Device, a novel solution for using the body’s own stem cells and healing capabilities to treat orthopedic…
Read MoreStimlabs® LLC Announces Launch of Relese® – A Uniquely Fenestrated Allograft for Chronic and Acute Wounds
Stimlabs LLC, (“StimLabs”), a leader in regenerative medicine, announced the launch of Relese, a fenestrated dehydrated complete human placental membrane (dCHPM) allograft for chronic and acute wounds. As the latest solution in StimLabs’ growing suite of dCHPM allografts, Relese provides a selective barrier with channels that allow wound fluid to drain while also protecting the wound…
Read MoreParagonix Technologies Receives FDA Clearance for BAROguard™ Donor Lung Preservation System
Paragonix Technologies, a leading organ transplant company, received US Food and Drug Administration (“FDA”) clearance for its next-generation donor lung preservation system, BAROguard™. The BAROguard™ System combines Paragonix’s existing advanced hypothermic preservation technology with automated continuous and active airway pressure control, ensuring that an optimal temperature range and a clinically recommended inflation pressure range for…
Read MoreFrom ATS to AI: Revolutionizing Your Resume Strategy for Success in the Job Search
In today’s competitive job market, crafting a resume that stands out from the crowd is essential. Artificial Intelligence (AI) has become a key part of the application process for candidates and employers alike and can be extremely beneficial for candidates in crafting a resume that is Automated Tracking System (ATS) friendly, as well as polished…
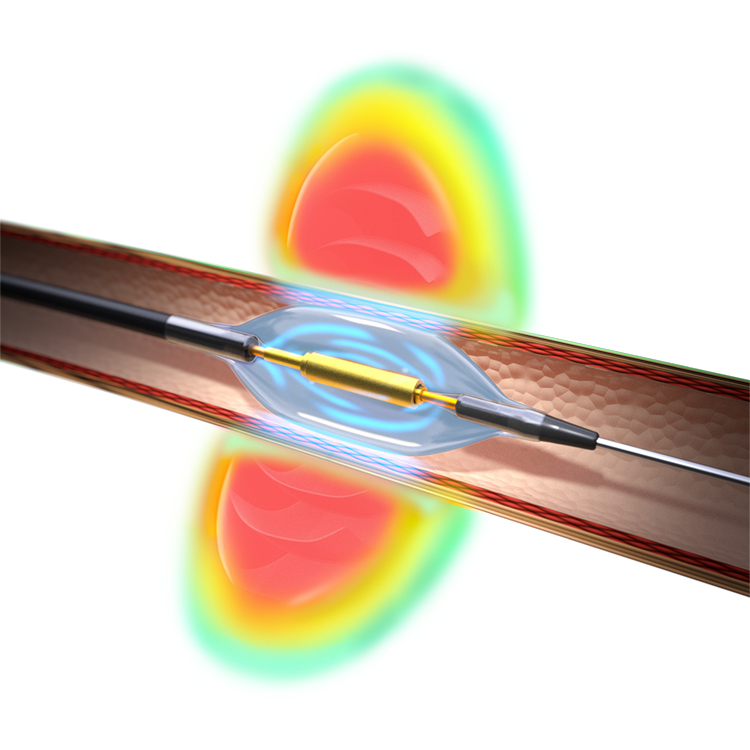
Read MoreRecor Medical and Otsuka Medical Devices Announce Positive Vote from the FDA Advisory Committee Meeting on the Paradise™ Ultrasound Renal Denervation System
Recor Medical, Inc. (“Recor”) and its parent company, Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”) announce the U.S. Food and Drug Administration (FDA) Circulatory Systems Devices Panel of the Medical Devices Advisory Committee met to discuss the pre-market approval application (PMA) for the Paradise™ Ultrasound Renal Denervation (RDN) system, indicated to reduce blood pressure in…
Read MoreLevita® Magnetics Wins FDA Clearance for Pioneering MARS™ System
Levita Magnetics, whose mission is to help more patients get access to better surgery, announced today it has received U.S. Food and Drug Administration (FDA) clearance for its MARS™ platform. The Levita MARS system is a first-of-its-kind minimally invasive surgical platform aimed at the high-volume abdominal surgery market. Harnessing the power of both magnets and…
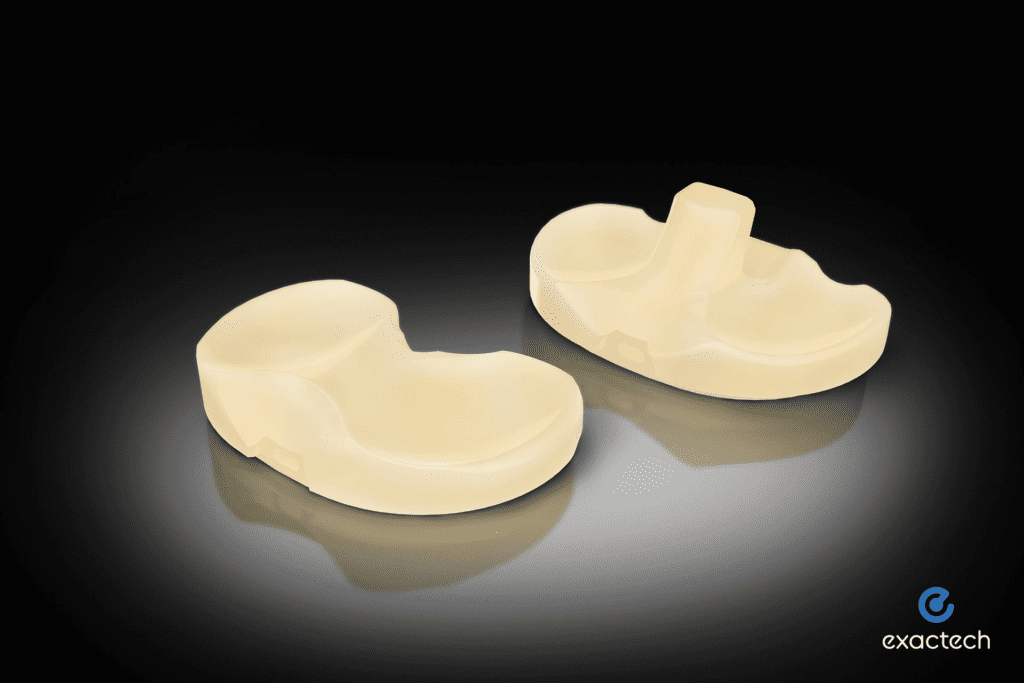
Read MoreExactech Announces FDA 510(k) Clearance for Advanced Activit-E™ Knee Replacement Polyethylene
Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced 510(k) clearance from the U.S. Food and Drug Administration for its new, advanced Activit-E™ polyethylene for the Truliant® knee replacement system. “After years of research and development in polyethylene, Activit-E represents a breakthrough achievement for Exactech,” said Adam…
Read MoreInBody Hits Milestone: 100 Million Tests Recorded Globally on Their LookinBody Web Platform
Highlighting a growing interest in body composition data, InBody is celebrating a long-anticipated milestone: 100 million tests taken globally on their body composition analyzers as of Friday, Aug. 4. “We’ve come a long way since InBody’s founding in 1996,” said Harry Yun, CEO of InBody USA. “The popularity of our tests is skyrocketing. Even the…
Read More