Archive for March 2020
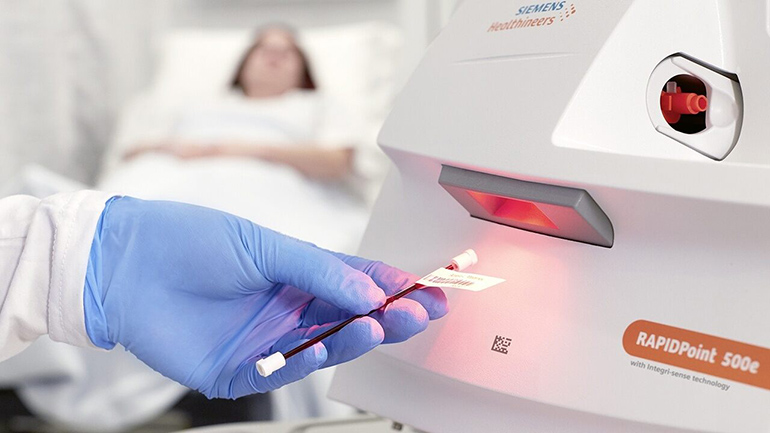
FDA Clears Siemens Healthineers RAPIDPoint 500e Blood Gas Analyzer Used for Critically Ill Patients in Acute Care Settings
Siemens Healthineers announced today that its latest critical care testing solution, the RAPIDPoint® 500e Blood Gas Analyzer, has received clearance from the U.S. Food and Drug Administration. The analyzer generates blood gas, electrolyte, metabolite, CO-oximetry, and neonatal bilirubin results, which are used to diagnose and monitor critically ill patients in the intensive care unit, operating…
Read MoreBattelle Announces Rapid Manufacturing of a System to Decontaminate N95 Respirator Masks
Battelle announced today that it has begun rapid manufacturing of a system to decontaminate N95 respirator masks and other medical protective equipment. Each Battelle CCDS Critical Care Decontamination System™ is capable of decontaminating up to 80,000 masks per day at full capacity. Because it is scalable, the system is capable of processing even more pieces…
Read MoreNorthwell Implements New On-site Laboratory Treatment Technology for COVID-19 Infected Medical Waste
To minimize the risk of spreading COVID-19, Northwell Health has become the first US health system to implement a new technology that breaks down medical waste from laboratory testing in a safe, environmentally friendly way. Developed by Irish-based Technopath Clinical Diagnostics, the Envetec 200 system simultaneously shreds and disinfects infectious waste using a patented destruction and disinfection…
Read MoreCardioQuip MCH-1000 Earns CE Mark Approval
CardioQuipTM, LLC, a medical device manufacturer focused on development and commercialization of patient temperature control and cardiovascular perfusion technology announced today it has received CE Mark approval for the MCH-1000TM Modular Cooler-Heater SeriesTM. The MCH-1000 Series of cooler-heaters is now available to European hospital systems fighting the COVID-19 pandemic. A cooler-heater device is used to regulate patient temperature…
Read MoreVERO Biotech Announces First Patient With COVID-19 Infection Complicating Pulmonary Hypertension Treated with GENOSYL DS, the First and Only FDA-Approved Tankless System for the Delivery of Inhaled Nitric Oxide
VERO Biotech LLC, an Atlanta, Georgia-based biotechnology company focused on saving lives, alleviating suffering, and improving the health economics of care, today announced that the first patient with COVID-19 infection complicating pulmonary hypertension has been treated with its proprietary inhaled nitric oxide (iNO) delivery system, GENOSYL® DS at home. The patient was treated under an emergency IND…
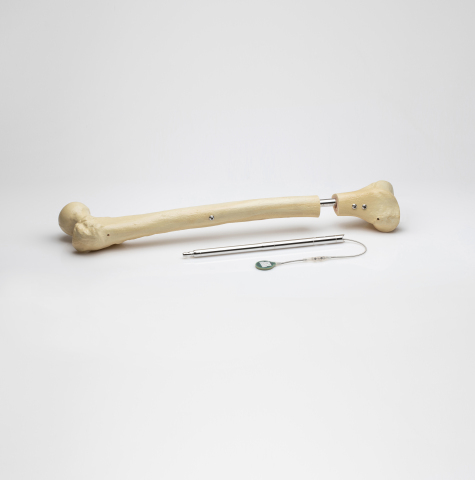
Read MoreOrthofix Completes Acquisition of FITBONE Limb Lengthening System
Orthofix Medical Inc., a global medical device company focused on musculoskeletal healing products, today announced it has completed the acquisition of assets associated with the FITBONE® intramedullary lengthening system for limb lengthening of the femur and tibia bones. The transaction also includes other potential applications of the technology which are in development, including the FITSPINE® system for…
Read MoreBiostage Announces IND Approval from FDA for its Lead Product Candidate Cellspan Esophageal Implant
Biostage, Inc., a bioengineering company developing next-generation esophageal implants, today announced that the U.S. Food and Drug Administration (FDA) has approved the Company’s Investigational New Drug application (IND) for the Cellspan Esophageal Implant (CEI) to treat patients with end-stage esophageal disease that require a segmental surgical resection to repair the diseased tissue. The FDA notified…
Read MoreBIOLASE Announces Regulatory Clearance of Laser Bacterial Reduction Therapy Indication for Epic Hygiene Laser
BIOLASE, Inc., the global leader in dental lasers, is pleased to announce the Epic Hygiene™ laser received regulatory clearance for Laser Bacterial Reduction (LBR) therapy indication from the Food and Drug Administration (FDA). This new indication now allows for the early management of periodontal disease utilizing laser light energy to reduce bacteria and thus decreasing…
Read MoreEchoNous, Inc. Announces CE Mark Approval for Its Healthcare AI KOSMOS Platform
EchoNous is very pleased to announce that its KOSMOS platform has been approved for CE Markets thanks to the unbending effort of its engineering, operations and regulatory teams. The company’s engineering team has developed a medical tool that, according to physician feedback, significantly increases provider confidence in bedside diagnostics and clinical decision-making with AI-assistance. The CE…
Read MoreNuclein Speeds Commercialization of First Ever Hand-Held PCR Test to Aid in COVID-19 Pandemic
Nuclein LLC announces plans to expedite the commercialization of its Nuclein™ Hand-Held PCR Test. The company’s disposable, all-in-one, self-test device for infectious disease diagnosis does not require technical expertise and provides battery-powered, sample-to-answer results in under one hour, without the need to mail in a sample. The company anticipates moving into product manufacturing soon, with the…
Read More