Archive for June 2023
Cresilon Receives First FDA Clearance For Human Use of Hemostatic Gel Technology
Cresilon, Inc. (“Cresilon”), a Brooklyn-based biotechnology company focused on hemostatic medical device technologies, today announced that it has been granted 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for Cresilon Hemostatic Gel™ (“CHG™”). This marks Cresilon’s first FDA clearance for human use, validating its revolutionary hemostatic gel technology and the company’s global mission to…
Read MoreUSMI Receives FDA Approval for New Robotic Surgery Device – The Canady Flex RoboWrist™
US Medical Innovations, LLC (USMI), a Biomedical and Life Science subsidiary of US Patent Innovations, LLC, announced today it has received FDA Approval for its Canady Flex RoboWrist™ to be used in open and laparoscopic procedures in the United States. The device is already approved and used in the Middle East, Europe, and Asia. The…
Read MoreCandela Medical Receives FDA Clearance for Vbeam 595 nm Pulsed Dye Laser, a Treatment for Port Wine Stains and Hemangiomas in Pediatric Cases
Candela Corporation (Candela), a leading global medical aesthetic device company headquartered in Marlborough, MA, today announced that the Vbeam Family of Pulsed Dye Lasers (PDL) has expanded its FDA cleared indications for use of the 595 nm wavelength to include the pediatric population (from birth – 21 years of age) for treatment of cutaneous capillary malformations, also…
Read MorePathKeeper Surgical Commercializes Spine Navigation System with First U.S. Cases
PathKeeper Surgical, a privately-held, Israel-based, medical technology company, dedicated to improving the health of individuals around the world suffering from all spinal issues requiring surgery, while making navigation-guided surgery available to any patient in all operating rooms, announces the first commercial use of the PathKeeper, 3D optical navigation system in the United States. The surgical debut of the…
Read MoreConformis Announces Definitive Agreement to be Acquired by restor3d for a Purchase Price of $2.27 Per Share in Cash
restor3d, Inc. and Conformis, Inc. (NASDAQ: CFMS) announced today that they have entered into a definitive merger agreement under which restor3d, a leading personalized 3D-printed orthopedic company, will acquire all outstanding shares of common stock of Conformis at $2.27 per share in cash, which represents an approximate 96 percent premium to the closing price of…
Read MoreNew FDA Clearance Makes Eyenuk the First Company with Multiple Cameras for Autonomous AI Detection of Diabetic Retinopathy
Eyenuk, a global artificial intelligence (AI) digital health company, and the leader in real-world applications for AI Eye Screening™ and AI Predictive Biomarkers™, has received U.S. Food and Drug Administration (FDA) clearance to use the Topcon NW400 retinal camera with its EyeArt AI system to automatically detect diabetic retinopathy (DR), adding to the already-cleared usage with Canon…
Read MoreOwlet Announces FDA-Clearance of the First Prescription Pulse Oximetry Sock for Infants
Owlet (NYSE: OWLT, “the Company”), the pioneer of smart baby monitoring, announces clearance from the U.S. Food and Drug Administration (“FDA”) of BabySat™, the first medical pulse-oximetry device featuring its advanced, wire-free sock design. Owlet is a leader in infant health data, having monitored more than 1 million babies, and with BabySat, combines its consumer-first…
Read MoreA Candid Conversation with Our CEO and Founder on Conquering Remote Work Challenges | Legacy MEDSearch
Written by: Melissa King and Paula Rutledge Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space.…
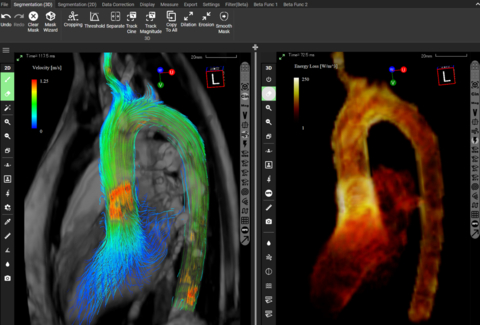
Read MoreFDA Approves Innovative 4D Flow MRI Blood Flow Analysis Software from Cardio Flow Design Inc.
The US Food and Drug Administration has approved the use of iTFlow® in blood flow analysis. The FDA approval is significant as it recognizes the safety and effectiveness of iTFlow® in analyzing blood flow through 4D Flow MRI data, with the potential to enhance diagnostic accuracy for patients with cardiovascular diseases and heart conditions. The…
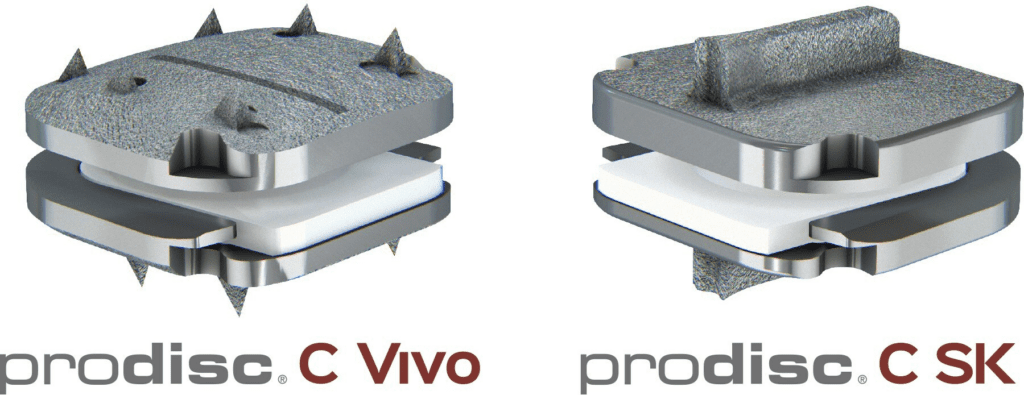
Read MoreCentinel Spine® Completes Enrollment in First-of-its-Kind 2-Level IDE Trial Evaluating prodisc® C Match-the-Disc™ Cervical TDR System
Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-proven total disc replacement technology platform in the world (prodisc®), today announced the completion of enrollment in a first-of-its-kind Investigational Device Exemption (IDE) study evaluating the Company’s prodisc C Vivo and prodisc C…
Read More