Archive for May 2020
AIROS Medical Launches New Compression Therapy Device and Garment System to Treat Breast Cancer Patients
AIROS® Medical, Inc., a medical technology manufacturer specializing in compression therapy products that treat cancer-related lymphedema and venous complications, today announced the launch of its updated AIROS 6 Sequential Compression Therapy device and Arm Plus garments following multiple regulatory approvals. AIROS received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its updated AIROS…
Read MoreGynesonics Receives FDA Clearance to Market Next Generation Sonata System 2.1
Gynesonics, a women’s healthcare company focused on the development of minimally-invasive solutions for symptomatic uterine fibroids, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its next generation Sonata® System 2.1 for Transcervical Fibroid Ablation (TFA). The Sonata technology platform integrates the first and only intrauterine…
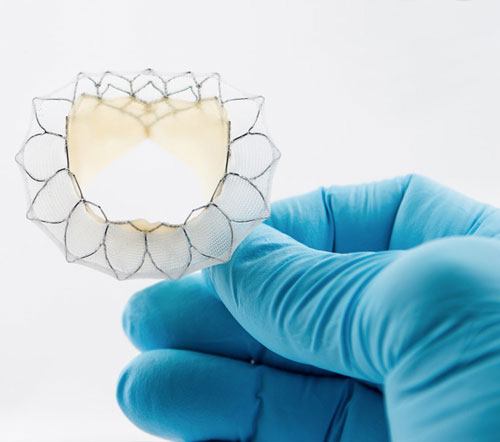
Read MoreNeovasc Has Filed for CE Mark for Tiara TA Transapical Mitral Valve Replacement System
Neovasc, Inc., a leader in the development of minimally invasive transcatheter mitral valve replacement technologies, and minimally invasive devices for the treatment of refractory angina, today announced that the Company has filed for CE Mark for its Tiara TA Transapical mitral valve replacement system. Mitral Valve disease is one of the most common forms of…
Read MoreCentinel Spine Announces FDA Approval for the Manufacturing Transfer of prodisc Technology
Centinel Spine, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced FDA approval for the manufacturing transfer of both the prodisc® C Cervical Total Disc Replacement and prodisc® L Lumbar Total Disc Replacement systems to new strategic vendors. The FDA approval for manufacturing transfer is a critical milestone for Centinel Spine as it allows the…
Read MoreSTERIS Announces Second On-site Decontamination Solution for Respiratory Masks
STERIS plc announced that the U.S. Food and Drug Administration (FDA) has issued another Emergency Use Authorization (EUA) for respirator decontamination. The EUA enables healthcare providers to decontaminate surgical N95 respirators by utilizing certain AMSCO Steam Sterilizers that have been upgraded with STERIS’s new “Decon” cycle. This EUA is the second authorization for STERIS to…
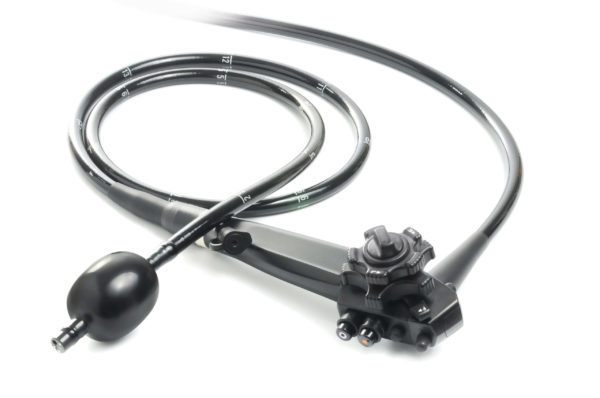
Read MoreSMART Medical Systems Receives FDA Clearance for Its G-EYE Colonoscope
SMART Medical Systems Ltd., a developer and manufacturer of innovative endoscopy products, announced that the FDA issued 510(k) clearance for its flagship product, the G-EYE® Colonoscope. The G-EYE® colonoscope is a standard colonoscope which SMART remanufactures by installing its proprietary G-EYE® balloon on the distal bending section of the colonoscope. During colonoscopy, the G-EYE® colonoscope is inserted in the…
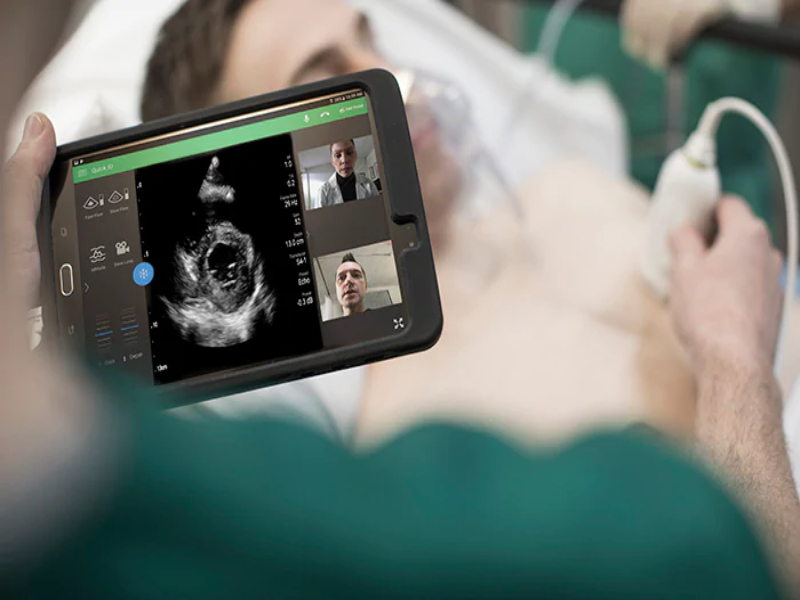
Read MorePhilips Receives FDA Clearance for the Use of its Ultrasound Portfolio to Manage COVID-19-Related Complications
Royal Philips, a global leader in health technology, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market a wide range of its ultrasound solutions for the management of COVID-19-related lung and cardiac complications. Handheld and portable ultrasound solutions in particular have become valuable tools for clinicians…
Read MoreFDA Approves Emergency Use of A&R Tarpaulins’ Patient Isolation Transportation Unit (PITU)
AR Tech, a division of A&R Tarpaulins Inc., an aerospace and medical equipment manufacturer, received emergency approval for the use of its Patient Isolation Transportation Unit (PITU) by the Food and Drug Administration. PITU is designed to temporarily isolate and transport patients with confirmed or suspected COVID-19 infection. “PITU is a game changer in this fight…
Read MoreSmith & Nephew launches new JOURNEY II Unicompartmental Knee System
Smith+Nephew, the global medical technology business, today announces the launch of its new JOURNEY II Unicompartmental Knee (UK) System. Built on the heritage of one of the most clinically successful partial knees,1,2 and paired with proprietary OXINIUM™ Technology, JOURNEY II UK is designed to help patients rediscover their normal life. JOURNEY II UK provides a highly personalized…
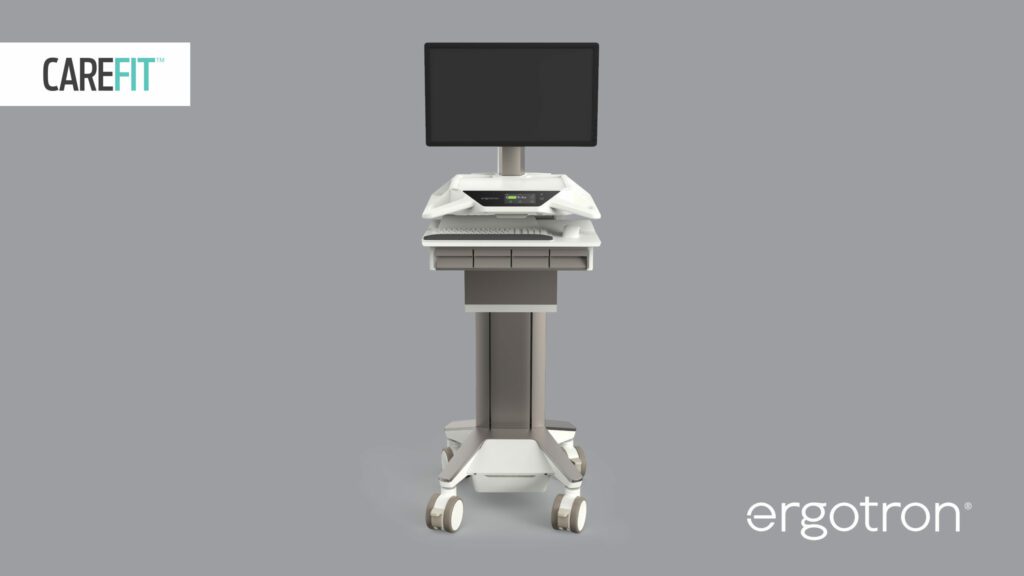
Read MoreNew Ergotron CareFit Pro Medical Cart Improves Caregiver Comfort, Efficiency
The new CareFit™ Pro Medical Cart from Ergotron can help improve caregiver comfort, streamline workflows and create better patient experiences. Developed based on years of research and insights from caregivers, CareFit Pro combines a highly mobile and adjustable design with a broad feature set to help caregivers be efficient and comfortable in their jobs. The cart also…
Read More