Archive for May 2018
Layoffs at IBM Watson Health Spark Concerns
IBM has laid off approximately 50 to 70 percent of staff this week in its Watson Health division, according to inside sources. The axe, we’re told, is largely falling on IBMers within companies the IT goliath has taken over in the past few years to augment Watson‘s credentials in the health industry. These include medical data…
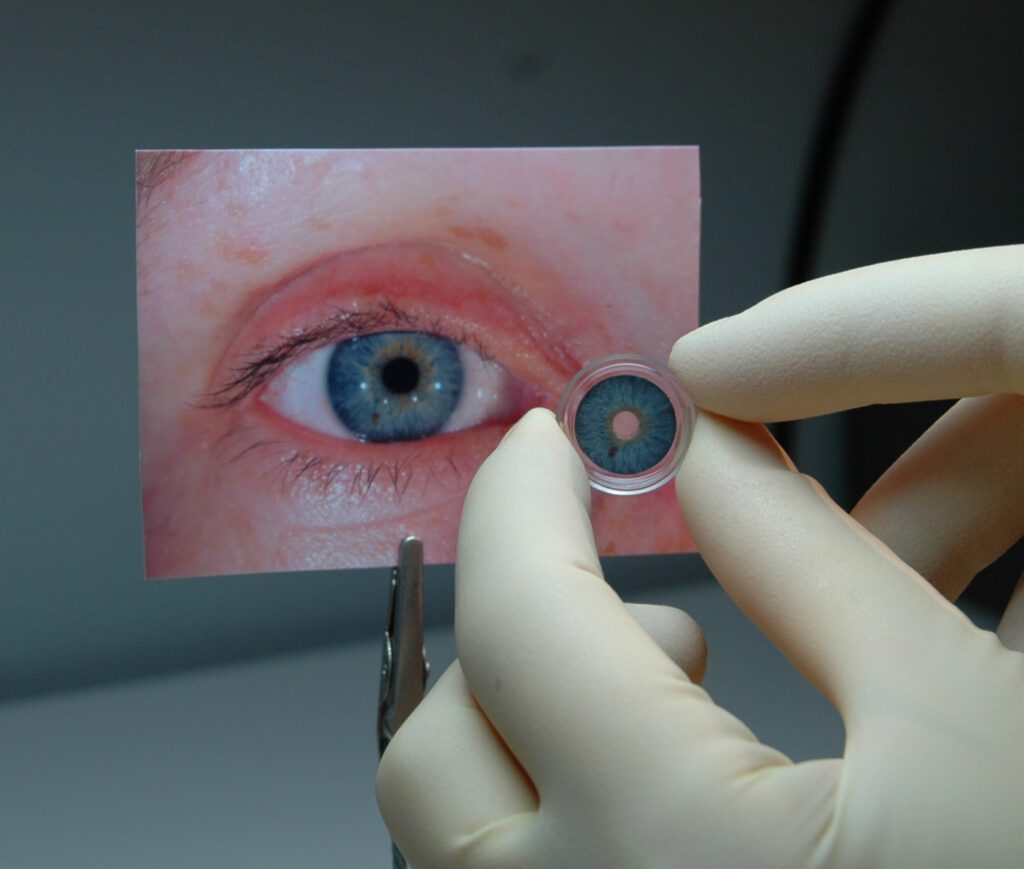
Read MoreFDA Approves First Artificial Iris
A German company has become the first to score FDA approval for a stand-alone prosthetic iris in the United States. The agency said Wednesday afternoon that it approved the CustomFlex Artificial Iris, made by Erlangen, Germany-based HumanOptics. The device, which is surgically implanted, is approved to treat adults and children whose iris is completely missing or damaged…
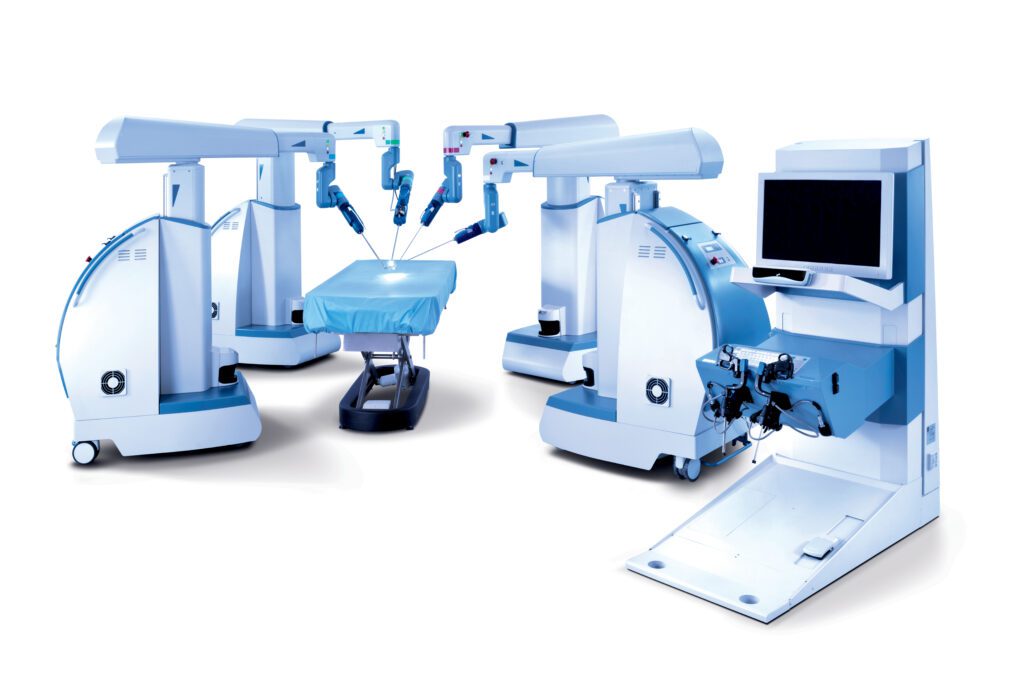
Read MoreTransEnterix Announces FDA Clearance for Expanded Indications for Senhance Surgical System
RESEARCH TRIANGLE PARK, N.C.–TransEnterix, Inc. (NYSE American:TRXC), a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced that the Company has received FDA 510(k) clearance for expanded indications of its Senhance Surgical System. The Company received FDA 510(k) clearance for laparoscopic inguinal hernia…
Read MoreClass I Recall Issued by FDA for Abbott’s HeartMate 3 Heart Pump
The FDA issued a Class I recall for Abbott’s HeartMate 3 left ventricular assist device due to possible twisting of the outflow graft that could result in a persistent low-flow alarm, which normally signals potential safety risks including low blood flow or clotting that could lead to serious injury or death. The regulatory agency recommends that patients experiencing…
Read MoreFDA Permits Marketing of First Autonomous Artificial Intelligence-Based Medical Device
On April 11, 2018, the U.S. Food and Drug Administration (“FDA”) permitted marketing of the first device to use artificial intelligence (“AI”) autonomously to detect a medical condition. The device, called IDx-DR, utilizes an AI algorithm to screen for diabetic retinopathy. The device is unique in that its results do not require additional review by…
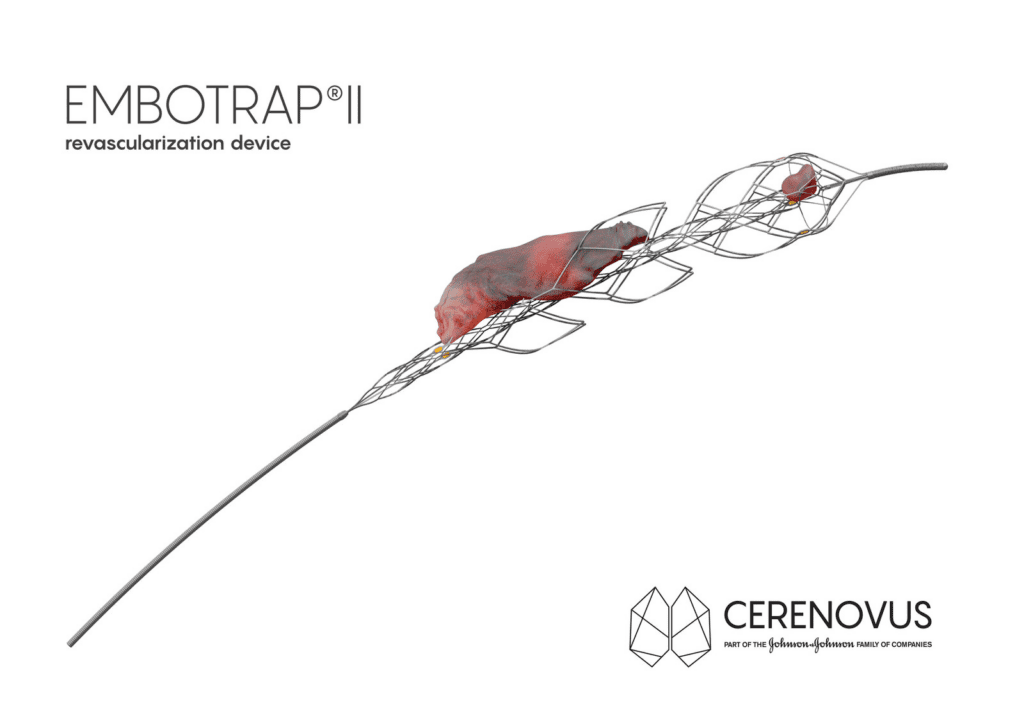
Read MoreNext Generation Stent Retriever Receives 510(k) Clearance
CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, announced today it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for EMBOTRAP II Revascularization Device, a next generation stent retriever used to capture and remove life-threatening blood clots from the brain following an ischemic stroke. Stroke strikes nearly 800,000 Americans…
Read More3D Printed Bone Segments for Foot and Ankle Fixation Receive 510(k) Clearance
Additive Orthopaedics, LLC., the leader in 3D printed orthopaedic foot and ankle devices, today announced that is has received FDA 510(k) clearance for its Patient Specific 3D Printed Bone Segments, to address internal bone fixation in the ankle and foot. According to Greg Kowalczyk, President of Additive Orthopaedics, “This is a tremendous milestone for orthopaedics and…
Read MoreMedical Device Companies Could Be Hurt By Chinese Tariffs
US medtech firms, including GE Healthcare (NYSE:GE) and the thriving Minnesota-based medtech sector, are concerned that tariffs on Chinese products proposed by President Trump could significantly affect their business, according to two reports released this week. GE Healthcare executives are worried that the tariffs could hurt the competitiveness of its products, including its magnetic-resonance imaging units which…
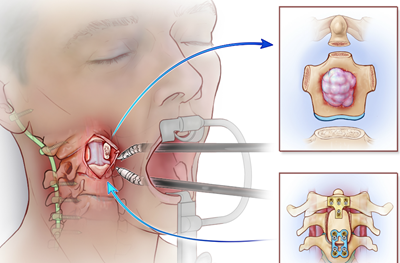
Read MoreFirst-in-World Robot-Assisted Spinal Surgery Performed by Penn Neurosurgeons and Otolaryngologists
Noah Pernikoff is back to his life in New York City after becoming the first patient in the world to undergo a complex three-part, robotic-assisted surgery. The robotic arms made it possible for the multidisciplinary team at Penn to successfully remove a rare tumor from Noah’s neck, where the skull meets the spine. The ground breaking…
Read MoreFirst Jellyfish-based biologics manufacturer receives funding
Jellagen, a pioneer in marine biotechnologies based in Wales, has closed a £3.8m funding round led by Newable Private Investing, the Development Bank of Wales, and Angel Investors. Newable Private Investing is one of the UK’s leading private investment groups, providing support to cutting-edge start-ups. As the first commercial manufacturer of next-generation jellyfish collagen for…
Read More