Archive for July 2020
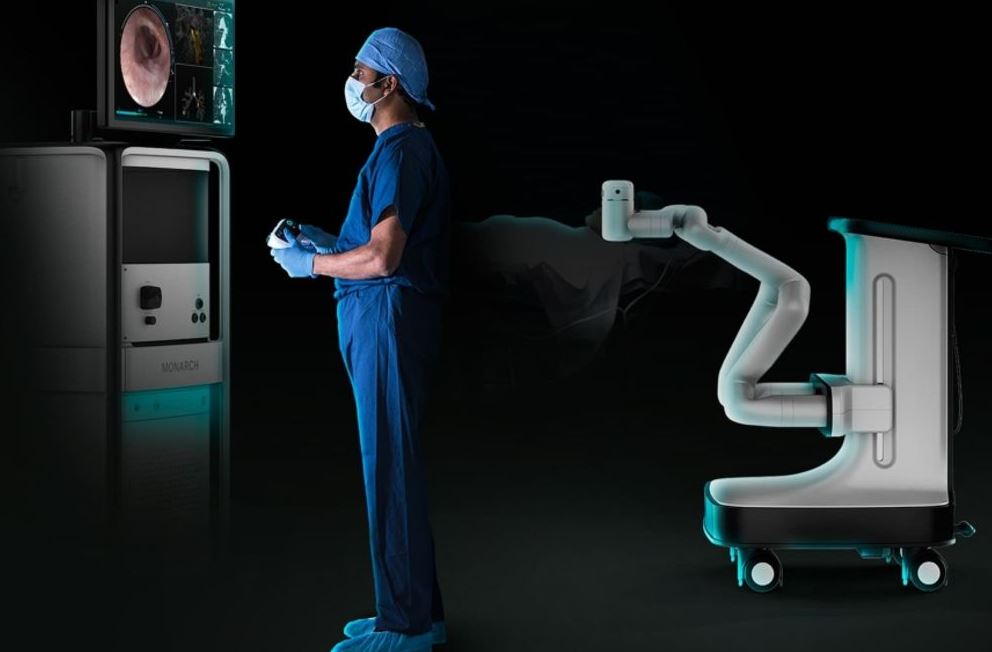
U.S. FDA Grants Ethicon Breakthrough Device Designation for Monarch-enabled NeuWave Microwave Ablation Technology
Ethicon, part of the Johnson & Johnson Medical Devices Company, announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for transbronchial microwave ablation technology using robotic-assisted bronchoscopy, which is currently under development. The Breakthrough Devices Program is a voluntary program for certain medical devices that provide for more effective treatment or…
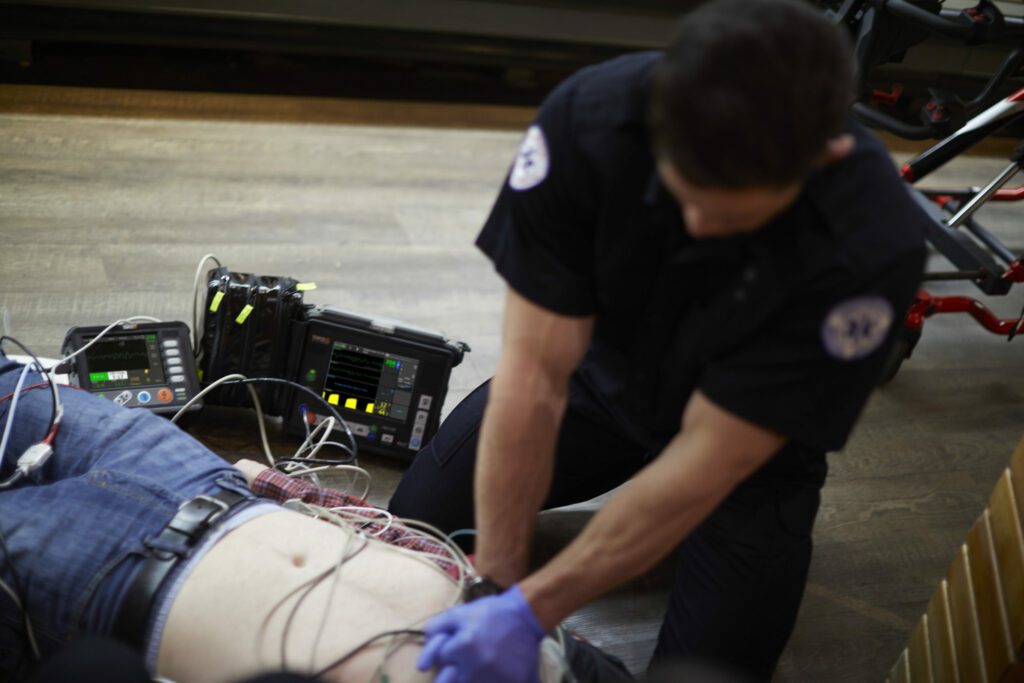
Read MorePhilips Wins FDA Clearance for EMS Remote Monitoring and Defibrillation Solution
Royal Philips, a global leader in health technology, today announced the launch of its remote monitoring and defibrillator solution (Tempus ALS) for pre-hospital settings in the U.S. The solution is a complete end-to-end system that combines innovative hardware and advanced software to expand the pre-hospital scope of care for first responders. The professional defibrillator (Tempus…
Read MoreFDA Clears Zebra Medical’s Breast Cancer AI for Spotting Suspicious Mammography Lesions
Zebra Medical Vision, the deep-learning medical imaging analytics company, announces today its sixth FDA 510(k) clearance for its mammography solution, HealthMammo, which has already received a CE mark. Zebra Medical’s algorithm empowers breast radiologists by prioritizing and identifying suspicious mammograms, providing a safety net for radiologists. The suspicious mammograms are identified faster and read earlier…
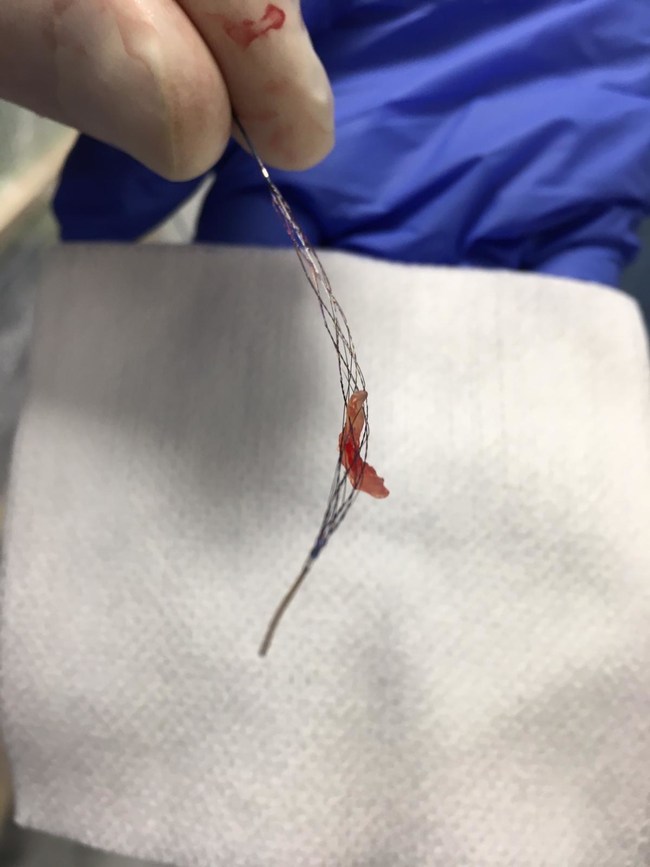
Read MoreRapid Medical Receives CE Mark for Stentriever
Rapid Medical, a company focused on the development of next generation neurovascular devices, has announced that it received CE Mark for TIGERTRIEVER XL. In addition, the first patients have been treated successfully with the device. The TIGERTRIEVER family of stentrievers are the first-ever adjustable, fully visible clot retrievers designed to treat ischemic stroke. Thousands of…
Read MoreArtio Medical Acquires Flow Forward Medical, Expanding Peripheral Vascular Portfolio
Artio Medical, Inc. (Artio) today announced it has acquired Flow Forward Medical, Inc. (Flow Forward), a medical device company developing innovative methods for establishing and maintaining high-quality vascular access sites to improve outcomes for hemodialysis patients. This stock-for-stock merger transaction in which Flow Forward merged with and into Artio was approved by the Board of…
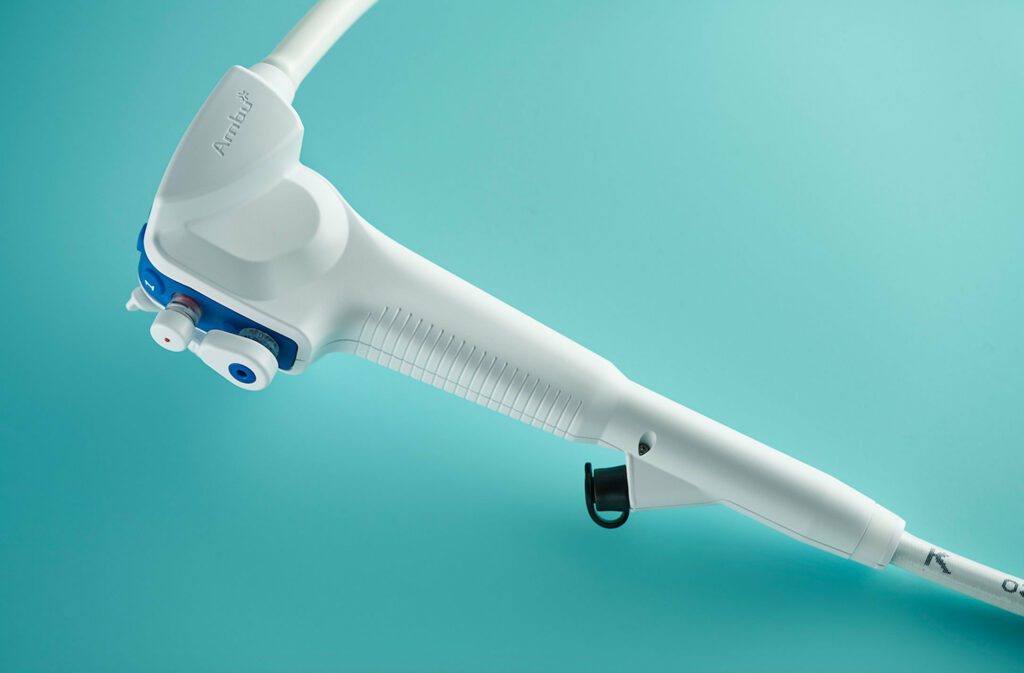
Read MoreAmbu Wins FDA Clearance for Single-use Duodenoscope Product
Ambu Inc., a rapidly growing medical device maker and pioneer of sterile, single-use endoscopes, announced today that the Ambu® aScope™ Duodeno has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). “At Ambu, we are determined to advance patient safety through innovative design of single-use devices, and we are excited to improve safety for the…
Read MoreFDA Approves Channel Medsystems’ Women’s Health Cryotherapy Device
Channel Medsystems, a company dedicated to bringing innovation to the delivery of women’s healthcare, today announced that the U.S. Food and Drug Administration (FDA) recently approved the newest Cerene Cryotherapy Device, a next-generation technology for the treatment of heavy menstrual bleeding in the office setting. Originally approved by the FDA in March 2019, Channel’s Cerene Device uses…
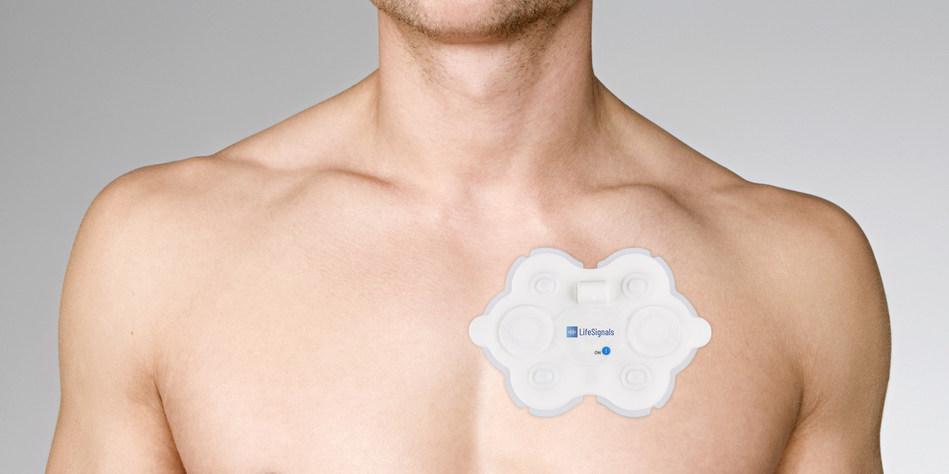
Read MoreLifeSignals Wireless Medical Biosensor Receives FDA Clearance
LifeSignals Group, today announced USFDA 510(k) clearance has been received for their LifeSignals ECG Remote Monitoring Patch Platform. The LifeSignals ECG Remote Monitoring Patch Platform is a wireless remote monitoring system intended for use by healthcare professionals for the continuous collection of Electrocardiography (ECG) and Heart Rate monitoring in ambulatory, hospital, home and healthcare settings.…
Read MoreFX Solutions Receives FDA 510k Clearances for Titanium Nitride Coated Humeral Heads and Glenospheres
FX received 510k clearances for their TiN (Titanium Nitride) Coated Humeral Heads and Glenospheres. The new addition of TiN coated humeral heads and glenospheres adds unique-to-market prostheses to the FX portfolio. The TiN coated humeral heads and glenospheres are identical to the current humeral heads and glenospheres that we offer but with the TiN coating applied. The TiN coated…
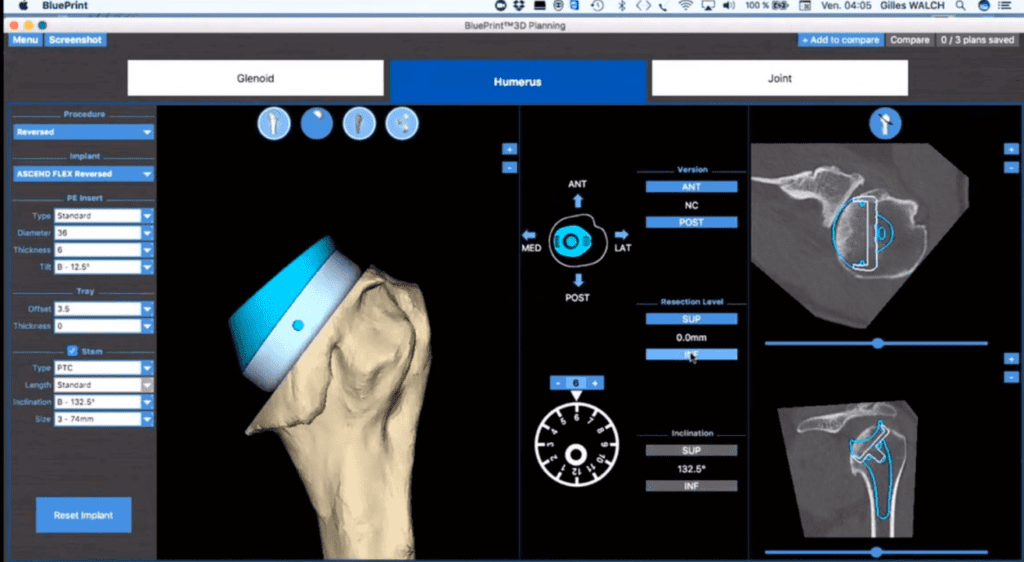
Read MoreMayo Clinic performs first shoulder arthroplasty with Wright Medical mixed reality tech
Wright Medical Group N.V. (NASDAQ: WMGI) today announced that the first shoulder arthroplasty procedure was performed using groundbreaking BLUEPRINT Mixed Reality Technology at Mayo Clinic’s campus in Rochester, Minnesota. Joaquin Sanchez-Sotelo, M.D., Ph.D, performed the procedure utilizing BLUEPRINT OR Visualization Mixed Reality software, which provides a 3-D holographic view of the patient’s pre-operative plan. Robert Palmisano, president and chief executive…
Read More