Archive for July 2020
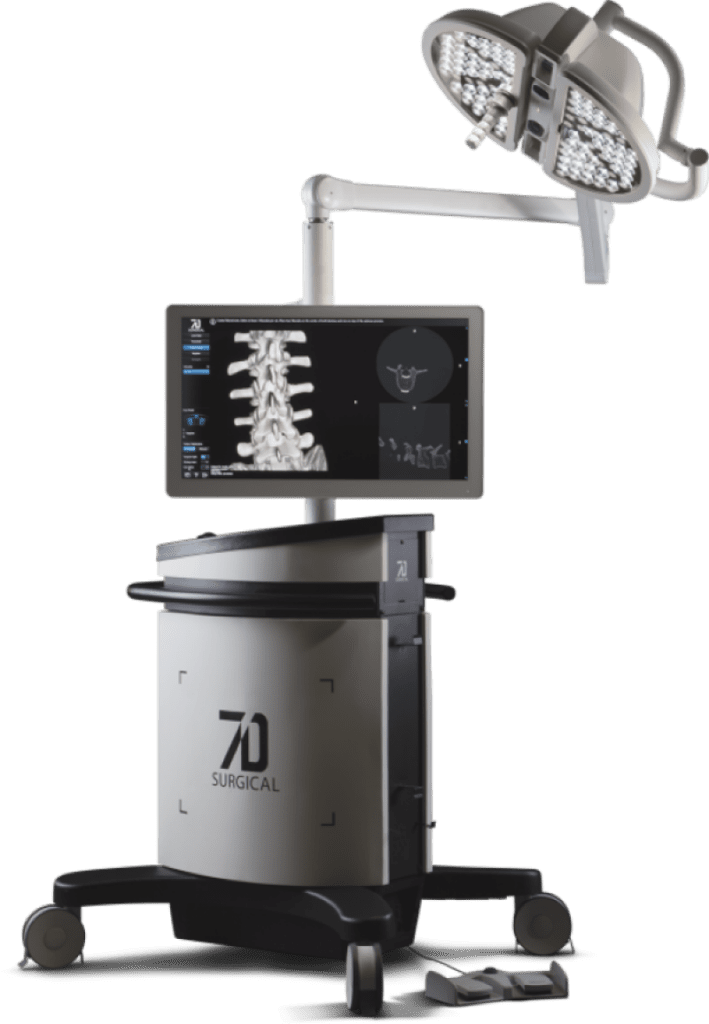
7D Surgical Wins CE Mark Certification for Machine Vision Image Guided Spine Surgery
7D Surgical announced today that it has achieved CE Mark certification for its innovative Machine-vision Image Guided Surgery (MvIGS) system for spine surgery. This achievement clears the way for 7D Surgical to commence commercialization efforts of its spinal platform across the European market and additional global regions. The 7D Surgical MvIGS System is the only…
Read MoreMedtronic to Acquire Medicrea
Medtronic plc (NYSE:MDT), a global leader in medical technology, and Medicrea, a pioneer in the transformation of spinal surgery through artificial intelligence, predictive modeling and patient specific implants, today announced that they have entered into a tender offer agreement for the acquisition of all outstanding shares of Medicrea. The friendly voluntary all-cash tender offer will…
Read MoreINSIGHTEC wins Medicare Coverage for MR-Guided Focused Ultrasound Platform
INSIGHTEC, a global medical technology innovator of incisionless surgery, announced today achievement of complete Medicare coverage across all 50 states for MR-guided focused ultrasound treatment of medication-refractory Essential Tremor. “INSIGHTEC is committed to expanding patient access for MR-guided focused ultrasound,” said Dee Kolanek, INSIGHTEC Vice President of Reimbursement. “The efforts of our team have made complete…
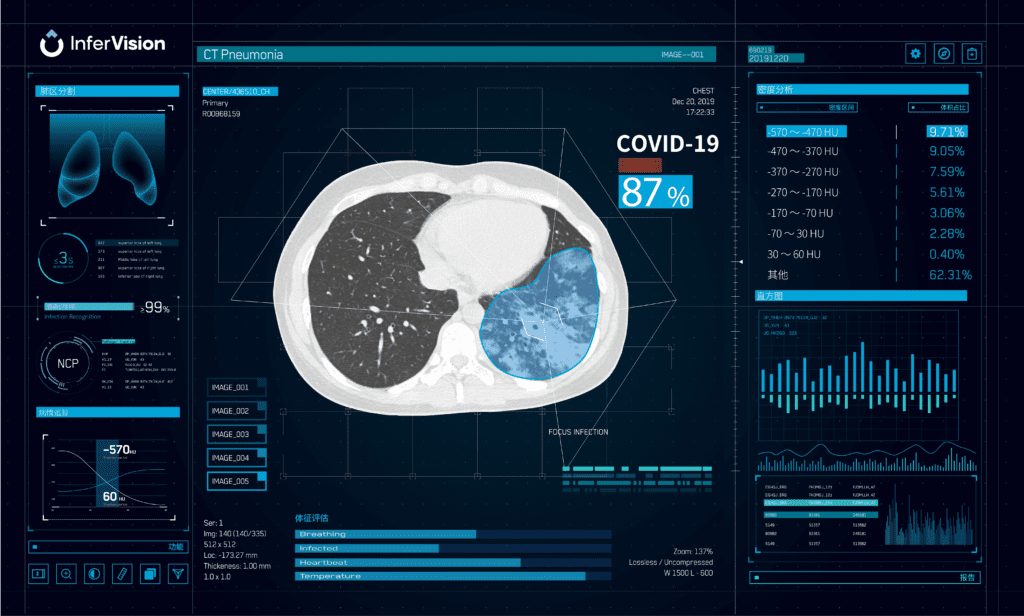
Read MoreFDA Green-lights Infervision’s Deep Learning Tool for Segmenting Lung CT Scans
Infervision is pleased to announce the U.S. Food and Drug Administration (FDA) 510(K) clearance of the InferRead Lung CT.AI product, which uses the state-of-the-art artificial intelligence and deep learning technology to automatically perform lung segmentation, along with accurately identifying and labeling nodules of different types. InferRead Lung CT.AI is designed to support concurrent reading and…
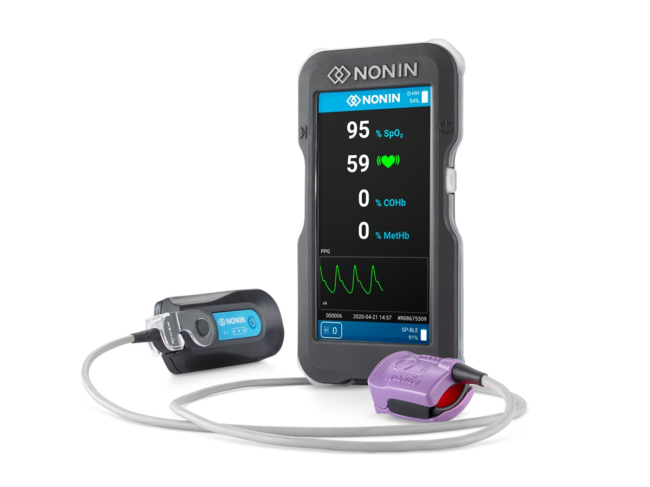
Read MoreFDA Clears Nonin Medical’s Flagship Product
Plymouth, Minn.-based Nonin Medical Inc. gained a U.S. FDA 510(k) clearance for its Co-Pilot wireless hand-held multiparameter system (H500). The system is expected to be used by first responders to evaluate various oxygenation and respiratory-related parameters in patients after incidents such as cardiac arrest, traumatic injury, carbon monoxide or smoke inhalation. It’s also expected to prove useful…
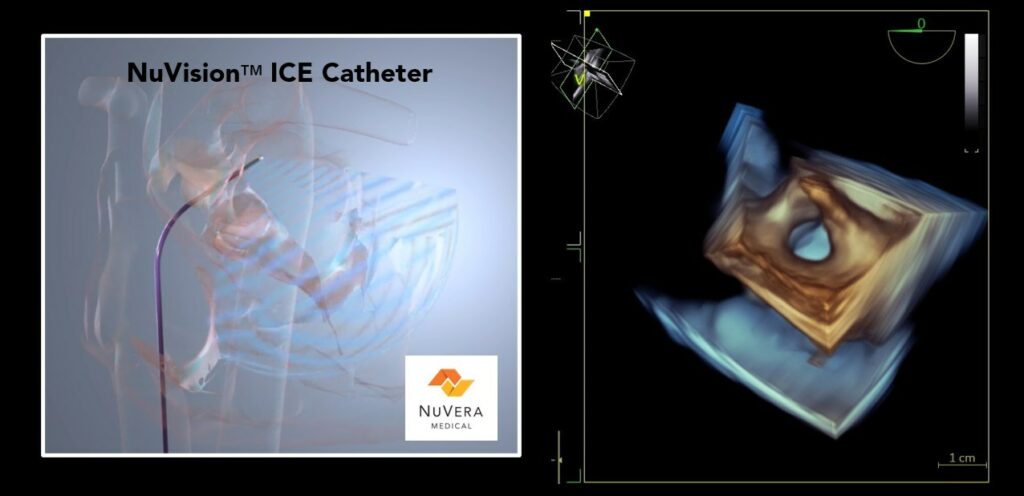
Read MoreNuVera Medical Announces Successful First-in-Human Use of NuVision ICE Catheter
NuVera Medical Inc. (NuVera), a portfolio company of Shifamed LLC, focused on enabling a new era in transcatheter cardiac interventions through the advancement of real-time 3D intracardiac echocardiography (4D ICE), announced today initiation of the company’s first-in-human clinical trial to evaluate the performance of its novel NuVision™ ICE Catheter. The first study participant was successfully treated this…
Read MoreUK Watchdog Skeptical of Stryker’s $4B Wright Medical Dea
The U.K. Competition and Markets Authority on Tuesday raised concerns that Stryker’s proposed $4 billion acquisition of Wright Medical Group could result in the medtech giant controlling 90% of Britain’s total ankle replacement prostheses market, leading to higher prices and less choice for hospitals and patients. CMA warned that the anti-competitive nature of the Stryker-Wright deal could have a negative…
Read MoreCVRx Announces Publication of BeAT-HF Clinical Study Results in the Journal of The American College of Cardiology
CVRx, Inc., a private medical device company, announced today that its BeAT-HF phase III randomized clinical trial results were published in the Journal of the American College of Cardiology (“JACC”). Results from the trial were used to obtain Premarket Approval (PMA) from the United States Food and Drug Administration (“FDA”) to market its BAROSTIM NEO…
Read More