Archive for January 2021
FDA Clears MULTIX Impact C Ceiling-Mounted DR System
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the MULTIX Impact C ceiling-mounted digital radiography (DR) system as well as the MULTIX Impact VA20, a new version of the established floor-mounted parent DR system. Economically priced, both systems expand access to high-quality imaging and enhance the patient experience. The MULTIX Impact…
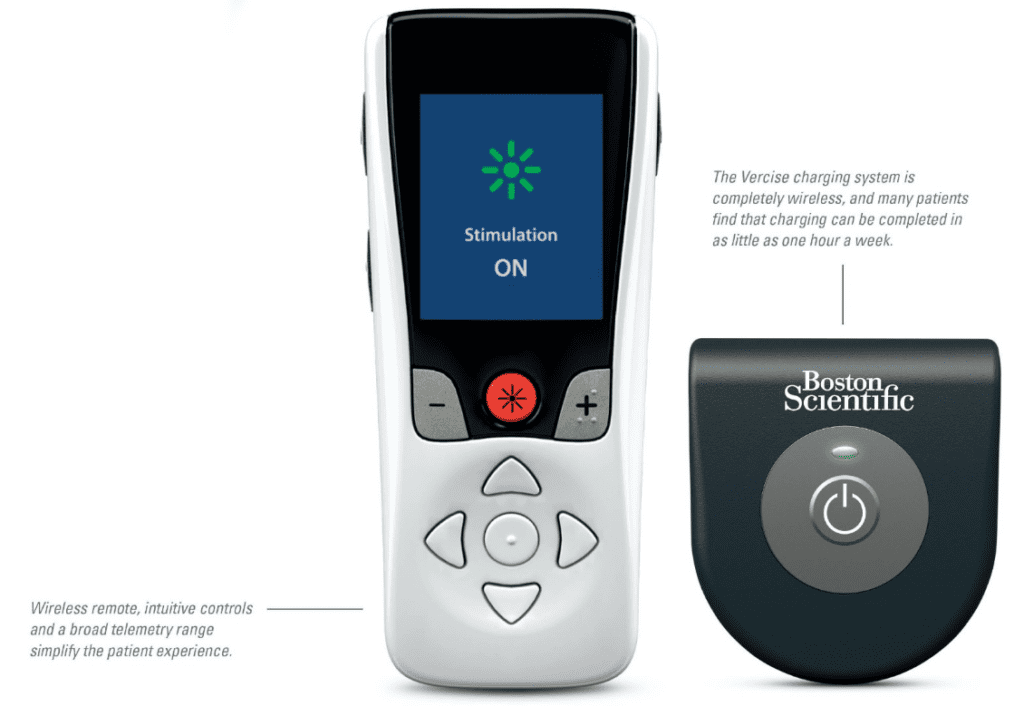
Read MoreFDA Approves Boston Scientific’s Vercise Genus Deep Brain Simulation System
Boston Scientific Corporation has received U.S. Food and Drug Administration approval of its fourth-generation Vercise Genus Deep Brain Stimulation (DBS) System. The portfolio, approved for conditional use in a magnetic resonance imaging (MRI) environment, consists of a family of Bluetooth-enabled, rechargeable and non-rechargeable, implantable pulse generators (IPGs) that power Cartesia Directional Leads, designed to provide…
Read MoreMedtronic Wins FDA Approval for DiamondTemp Ablation System
Medtronic, the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) approval of the DiamondTemp Ablation (DTA) system which treats patients with recurrent, symptomatic paroxysmal atrial fibrillation (AF) and who have been unresponsive to drug therapy. The DiamondTemp system is the first FDA-approved, temperature-controlled, irrigated radiofrequency (RF) ablation…
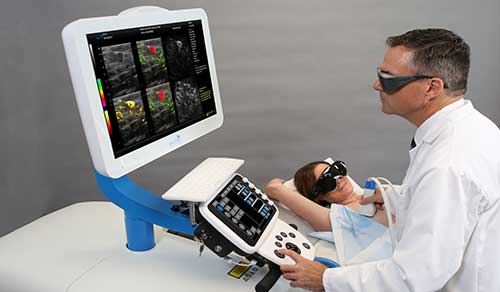
Read MoreFDA Approves Seno Medical’s Breast Lesion Diagnostic Technology
The Center for Devices and Radiological Health (CDRH) of the US Food & Drug Administration (FDA) has granted Texas-based Seno Medical Instruments, Inc. (Seno) premarket approval (PMA) for its groundbreaking diagnostic breast cancer imaging technology that helps physicians better differentiate between benign and malignant breast lesions. The company’s Imagio Breast Imaging System uses non-invasive opto-acoustic ultrasound…
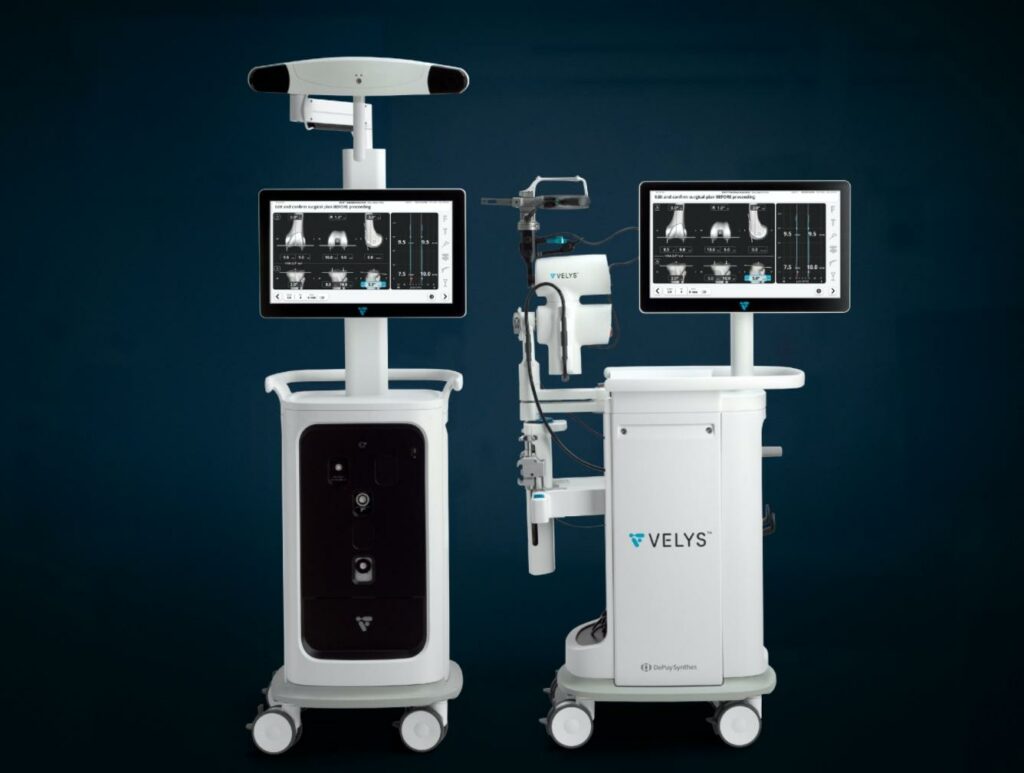
Read MoreDePuy Synthes Receives 510(k) FDA Clearance for VELYS Robotic-Assisted Solution Designed for Use with the ATTUNE Total Knee System
Today, The Johnson & Johnson Medical Devices Companies announced that DePuy Synthes has received 510(k) clearance from the U.S. Food and Drug Administration for the VELYS Robotic-Assisted Solution designed for use with the ATTUNE Total Knee System and its cleared indications for use. It will become part of the broader VELYS Digital Surgery Platform of…
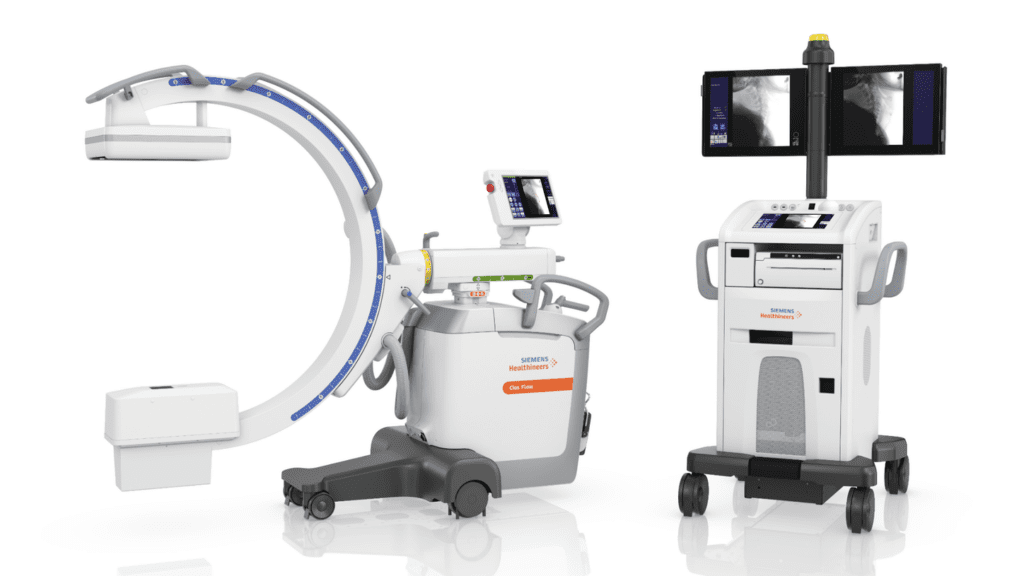
Read MoreFDA Clears Siemens’ Cios Flow Mobile C-arm System
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the Cios Flow, a mobile C-arm designed for use by multiple disciplines in the operating room (OR) – including orthopedics, trauma surgery, spinal surgery, vascular surgery, and pain therapy – to increase the ease and efficiency of everyday imaging workflows for surgical interventions.…
Read MoreSTERIS Spending $4.6B to Acquire Cantel Medical
STERIS plc (NYSE: STE) (“STERIS” or the “Company”) and Cantel Medical Corp (NYSE:CMD) (“Cantel”) today announced that STERIS has signed a definitive agreement to acquire Cantel, through a U.S. subsidiary. Cantel is a global provider of infection prevention products and services primarily to endoscopy and dental Customers. Under the terms of the agreement, STERIS will…
Read MoreItamar Medical Acquires Technology and Assets of Spry Health
Enables Itamar to leverage Spry’s existing FDA-cleared technology to bring to market the first device for continuous remote patient monitoring (RPM) of sleep apnea Adds the capability to monitor the longer-term accumulated disease burden of sleep apnea to complement the single night diagnostics commonly used today, including the Company’s existing WatchPAT™ device Paves the way…
Read MoreLocate Bio’s CognitOss Lands FDA Breakthrough Device Designation
Locate Bio, an orthobiologics focused regenerative medicine company, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to CognitOss, currently in development for the treatment of chronic osteomyelitis. The FDA Breakthrough Device Program is intended to help patients receive more timely access to certain technologies that have…
Read MoreHologic to Makes Second Major Acquisition of 2021, Acquires Biotheranostics for $230M
Hologic, Inc. (Nasdaq: HOLX), a global leader in women’s health, announced today that it has agreed to acquire Biotheranostics, Inc., a privately held, commercial-stage company that provides molecular diagnostic tests for breast and metastatic cancers, for approximately $230 million, subject to working capital and other customary closing adjustments. “Acquiring Biotheranostics enables us to jump-start our…
Read More