Archive for February 2019
Axilum Wins 510(k) Clearance for Robotic TMS System
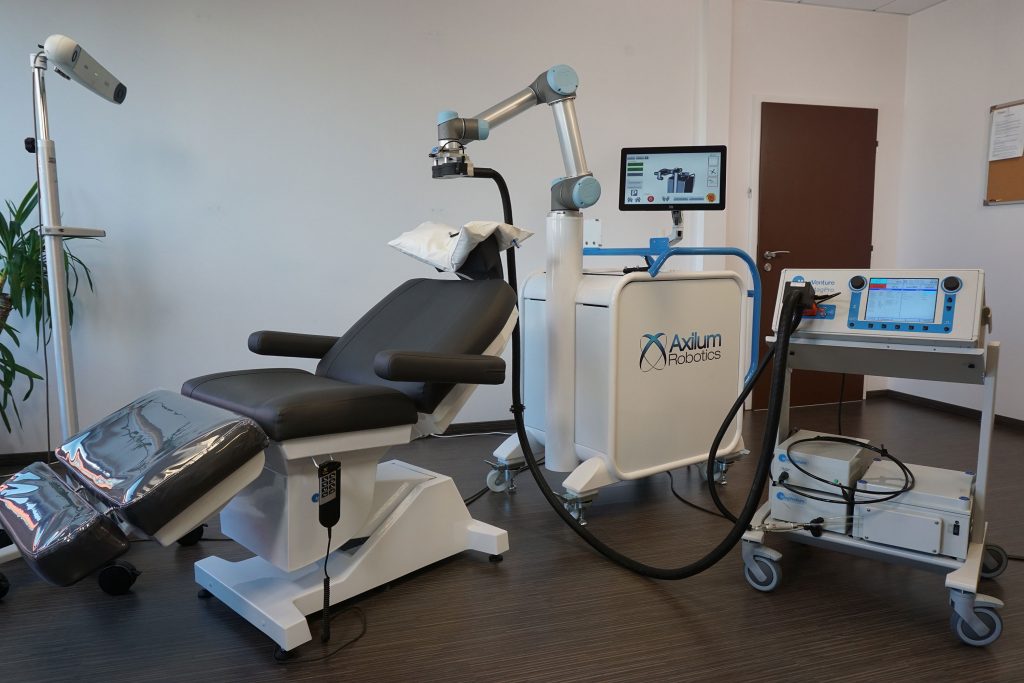
Axilum Robotics, which specializes in the development of medical robots, announced that two weeks after the CE mark, it has received 510(k) clearance from the U.S. Food and Drug Administration to market the TMS-Cobot TS MV for the spatial positioning and orientation of the treatment coil of the MagVenture TMS Therapy system. The company successfully developed…
Read MoreIntuitive Surgical Gains FDA Clearance for Lung Cancer Biopsy Robot
Intuitive Surgical Inc. said last week that the U.S. Food and Drug Administration has cleared its Ion system. The robotic endoluminal system is designed to enable doctors to conduct minimally invasive biopsies deep within the lung. Ion includes an articulating robotic catheter with an outer diameter of only 3.5 mm that is able to move…
Read MoreMassive Medtronic Pacemaker Recall is Class I Says FDA
The FDA has slapped the most serious designation on a recall of approximately 156,957 Medtronic dual-chamber pacemakers. The Fridley, Minn.-based company issued a recall in January on pacemakers with model names Adapta, Versa, Sensia, Relia, Attesta, Sphera, and Vitatron due to a software error that can result in a lack of pacing. Patients and physicians cannot predict whether and when this…
Read MoreJ&J Acquires Robotic-Surgery Firm Auris for $3.4 Billion
Focused on creating the next frontier of surgery, Johnson & Johnson, today announced that Ethicon, Inc., entered into a definitive agreement to acquire Auris Health, Inc. for approximately $3.4 billion in cash. Additional contingent payments of up to $2.35 billion, in the aggregate, may be payable upon reaching certain predetermined milestones. Auris Health is a…
Read MoreSmith & Nephew Discussing $3B NuVasive Acquisition
Smith & Nephew is in discussions to pick up spine-focused medtech developer NuVasive Inc. in a deal that could be worth more than $3 billion, according to a report from the Financial Times released late last week. The companies have not officially commented on the possible acquisition. NuVasive has a market valuation of approximately $2.6 billion, according to a Reuters report, with the alleged…
Read MoreFish-Skin Wound Care Company Gets Swiss Approval, Makes Acquisition
Kerecis, the company pioneering the use of fish skin in tissue regeneration, today received a notice from Swiss healthcare authorities that the company’s lead product, Kerecis Omega3 Wound, will be reimbursed in Switzerland. The company will also acquire the Swiss life-science company Phytoceuticals. The aim of the acquisition is to establish sales in Switzerland and strengthen Kerecis’ position…
Read MoreSirakoss Wins CE Mark for Osteo3 Bone Graft Substitute
SIRAKOSS Ltd, a developer of nanosynthetic bone graft substitutes, announced today it has been granted CE Mark clearance in the European Union (EU) for Osteo3, a novel nanosynthetic bone graft substitute designed to improve patient healing, offering surgeons a more advanced solution for repairing bone fractures. Synthetic bone grafts are used to fuse bones together…
Read More