Archive for February 2020
U.S. FDA Grants PAVmed Subsidiary, Lucid Diagnostics, Breakthrough Device Designation for its EsoGuard Esophageal DNA Test
PAVmed Inc., a highly differentiated, multiproduct medical device company, today announced that the Company’s majority owned subsidiary, Lucid Diagnostics Inc. (“Lucid”), has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its EsoGuard™ Esophageal DNA Test on esophageal samples collected using its EsoCheck™ Cell Collection Device in a prevalent well-defined group…
Read MoreArizona Startup Emagine Solutions Technology Receives FDA 510(k) Clearance of its VistaScan Mobile Ultrasound
Emagine Solutions Technology, an award-winning medical software device company located in Tucson, announced that it has received clearance from the U.S. Food & Drug Administration (“FDA”) to market its VistaScan mobile ultrasound platform. The VistaScan platform transforms a clinician’s cell phone or tablet into a portable ultrasound solution. The system consists of compatible FDA cleared ultrasound probes…
Read MoreEndotronix Announces Enrollment of the First Patients in the PROACTIVE-HF Pivotal Trial
Endotronix, a digital health and medical technology company dedicated to advancing the treatment of chronic heart failure (HF), today announced the enrollment of the first two patients in the PROACTIVE-HF pivotal trial. The trial is a pre-market investigational device exempt (IDE) study evaluating the safety and efficacy of the Cordella™ Pulmonary Artery (PA) Pressure Sensor…
Read MoreWaveGuide Launches World’s First Portable NMR Device: the WaveGuide Formµla
WaveGuide Corporation, an innovator of portable micro NMR (Nuclear Magnetic Resonance) instruments, today launched its new WaveGuide Formµla™, the world’s only battery-powered, compact scientific instrument that performs rapid screening and diagnostics of solid and liquid substances spanning an array of markets and applications. The WaveGuide Formµla™ micro NMR delivers performance as good or better than…
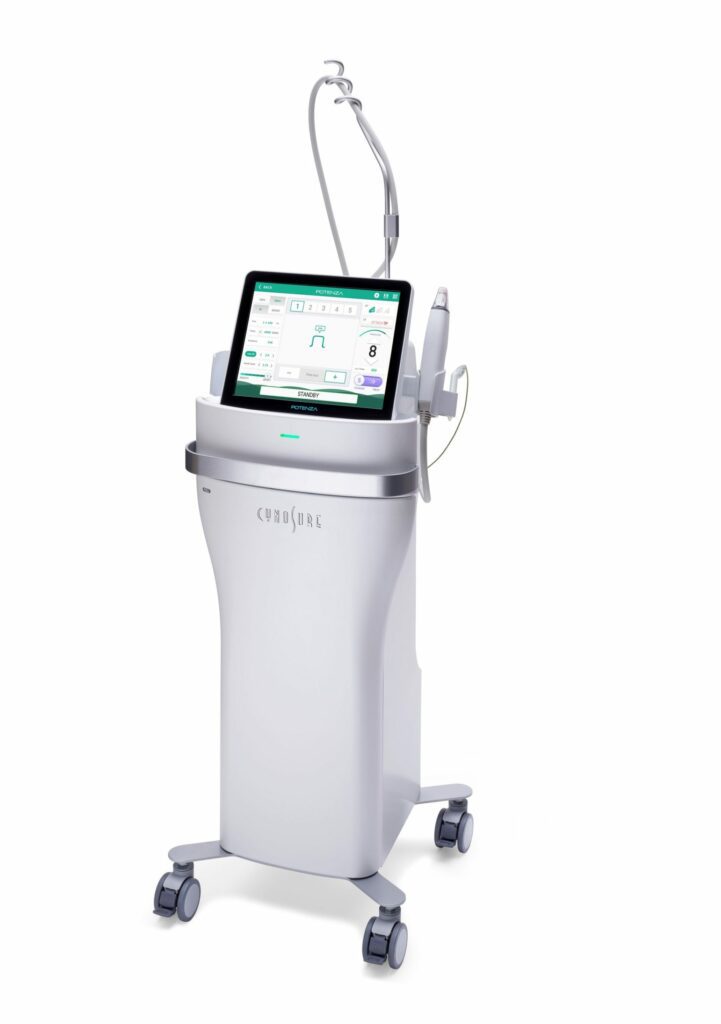
Read MoreCynosure Launches Potenza Radiofrequency Microneedling Device Expanding Company’s Growing Skin Revitalization Portfolio
Cynosure, the global leader in medical aesthetics, announced today the U.S. Food and Drug Administration (FDA) clearance of the Potenza™ radiofrequency (RF) microneedling device, the first and only FDA-cleared four-mode RF microneedling device offering clinicians unrivaled versatility and personalized treatments for patients. The new standard in RF microneedling, the Potenza device’s four modes (monopolar or…
Read MoreReadCoor, Inc. Unveils True Spatial Sequencing Platform to Drive Groundbreaking Insights into Immuno-oncology, Neuroscience, and Infectious Disease
ReadCoor, Inc., a company leading true multi-omic spatial sequencing, today unveils its first product line, including multi-omic spatial sequencing assays and the RC2 instrument. The platform is powered by ReadCoor’s proprietary FISSEQ (Fluorescent in situ Sequencing) technology, which combines the massive multiplexity of next-generation sequencing (NGS) and high-resolution tissue imaging. The fully integrated platform is now available for use by researchers…
Read MoreImmunoPrecise Launches Coronavirus Vaccine and Therapeutic Antibody Program
ImmunoPrecise Antibodies Ltd (the “Company”), a provider of best-in-class therapeutic antibody discovery capabilities for the global industry, announces its commitment to the development of innovative vaccines against the new coronavirus originating in Wuhan, China (SARS-CoV-2) as well as coronavirus-neutralizing antibodies, addressing both prophylactic and therapeutic measures to fight the virus and its associated disease, COVID-19. IPA’s Rapid Research…
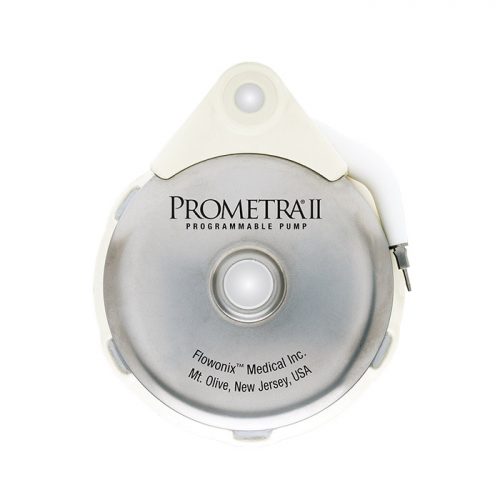
Read MoreFlowonix Receives FDA Approval to Market Prometra II Pump for use with Intrathecal Baclofen
Flowonix Medical, Inc. today announced United States Food and Drug Administration (FDA) approval to market the Prometra II Programmable Pump System for use with intrathecal baclofen. Flowonix introduced the Prometra II 40mL pump to the US market in November 2019, providing patients and clinicians a choice between 20 mL and 40 mL capacities when choosing an…
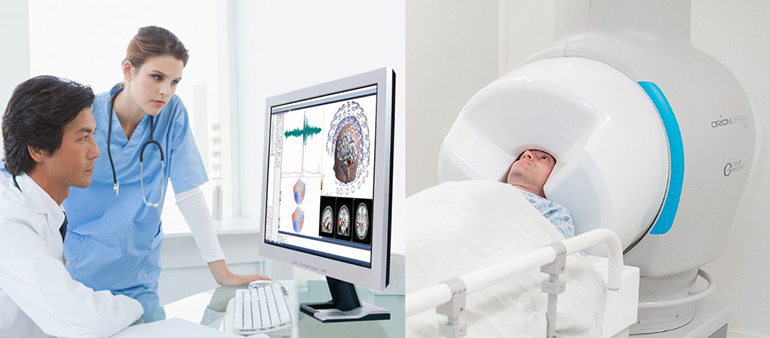
Read MoreCompumedics Announces Milestone US FDA Approval for the Orion Lifespan MEG
Compumedics Limited is pleased to announce that on February 14, 2020, it received 510(K) clearance from the US Food and Drug Administration (FDA) for its Orion LifeSpan™ Magnetoencephalography (MEG) single Dewar system. This news follows the successful installation and first phase commissioning of the single Dewar Orion LifeSpan™ MEG at Barrow Neurological Institute (BNI) in Phoenix,…
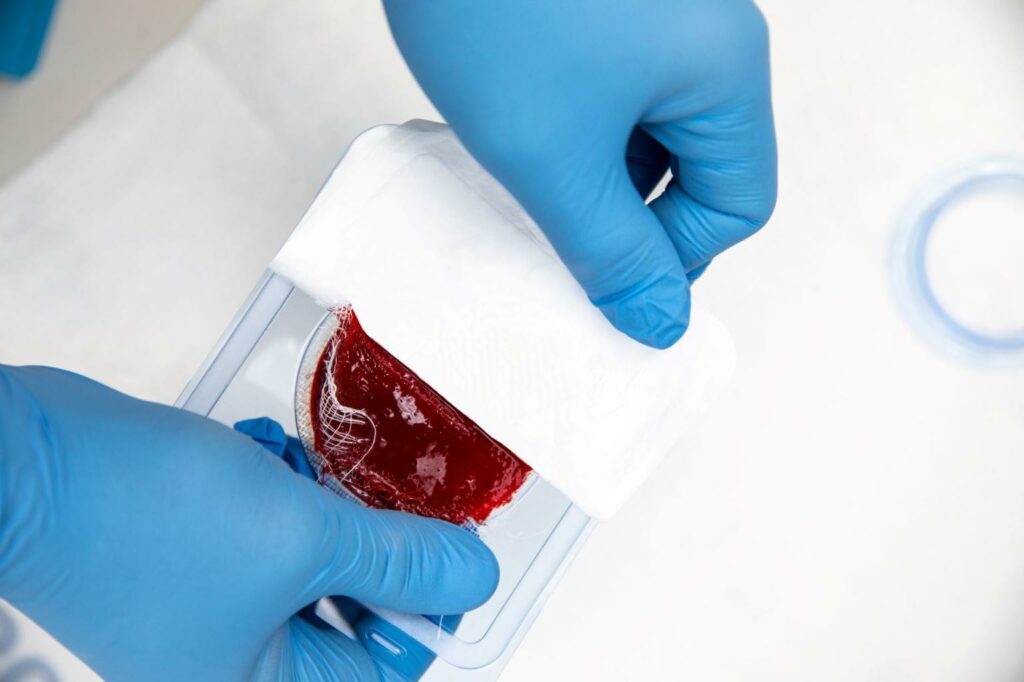
Read MoreRedDress Announces Launch of ActiGraft, FDA-Cleared for the Treatment of Chronic and Acute Wound Types
RedDress, a privately held, Israel and U.S. based company, today announced the U.S. launch of ActiGraft, the first wound treatment that transforms – in real time – a patient’s blood into an autologous whole blood clot tissue. Once applied, ActiGraft serves as a protective covering, biologic scaffold and wound microenvironment to promote the natural wound healing processes…
Read More