Archive for November 2018
Itamar Medical Reports Record Third Quarter Results
Itamar Medical, a company that develops, manufactures, and markets non-invasive diagnostic medical devices for sleep apnea with a focus on the cardiology market, today reported financial results for the third quarter of 2018. “We are pleased with the solid increase in third-quarter revenues, which reflects the growing recognition among U.S. cardiologists of the benefits that…
Read MoreFDA Releases Plan to Modernize the 510(k) Pathway
The FDA today released plans for updating its 510(k) clearance pathway, including a push to move away from using predicate devices over 10 years old and the creation of a new alternative 510(k) pathway that will allow approval based on objective safety and performance criteria. In an official posting, FDA head Dr. Scott Gottliebb and…
Read MoreBoston Scientific to Acquire BTG for $4.2 Billion
Boston Scientific today announced it has reached an agreement on the terms of a recommended offer to acquire BTG plc., a company headquartered in the United Kingdom, which develops and commercializes products used in minimally-invasive procedures targeting cancer and vascular diseases, as well as acute care pharmaceuticals. The transaction has been unanimously approved by the boards…
Read MoreBoston Scientific to Restructure, Expects $200M in Cut Costs
Boston Scientific said yesterday that it’s planning a three-year restructuring program that’s slated to cost at least $200 million. The Marlborough, Mass.-based medical device maker said it expects to maintain roughly the same number of workers, although there will be some churn as new jobs are created. “[T]he company does expect some employee attrition and…
Read MoreItamar Medical Gets Favorable CMS Reimbursement Decision for WatchPAT Technology
Itamar Medical Ltd. today announced the release of the 2019 Fee Schedule from the U.S. Centers for Medicare & Medicaid Services (CMS) that should support broad use of its WatchPAT technology. WatchPAT is the only product on the market today that uses Peripheral Arterial Tone technology, providing cardiologists and sleep physicians in the United States…
Read MoreFDA Approves Implantable Glucose Monitor Insertion/Removal by Non-Physicians
Senseonics Holdings, Inc., a medical technology company focused on the development and commercialization of a long-term, implantable continuous glucose monitoring (CGM) system for people with diabetes, today has announced that the Eversense® Continuous Glucose Monitoring (CGM) System has received FDA approval for qualified health care providers to be trained and certified to provide patients with the…
Read MoreFDA Clearance for Artificial Intelligence Upgrade to Ninepoint’s Imaging System
NinePoint Medical, Inc., a transformative medical device company pioneering the use of a real-time imaging platform for gastrointestinal applications, announced today that it has received U.S. Food and Drug Administration (FDA) clearance to market its Intelligent Real-time Image Segmentation™ (IRIS) software upgrade for its flagship product, the NvisionVLE Imaging System. IRIS, an artificial intelligence-based platform…
Read MoreRTI Surgical to Acquire Paradigm Spine for $300M
RTI Surgical, Inc., a global surgical implant company, and Paradigm Spine, LLC, a leader in motion preservation and non-fusion spinal implant technology, today announced that they have entered into a definitive agreement whereby RTI will acquire all outstanding equity interest of Paradigm Spine in a cash and stock transaction valued at up to $300 million,…
Read MoreIs the Appendix the Key to Parkinson’s Disease?
Parkinson’s disease, a degenerative neurological disorder that impairs brain cells and causes movement problems, could have its origins in the appendix, a new study suggests. The vestigial organ, the researchers say, could be the source of proteins that can find their way to the brain and once there, extend a deadly grip on nerve cells.…
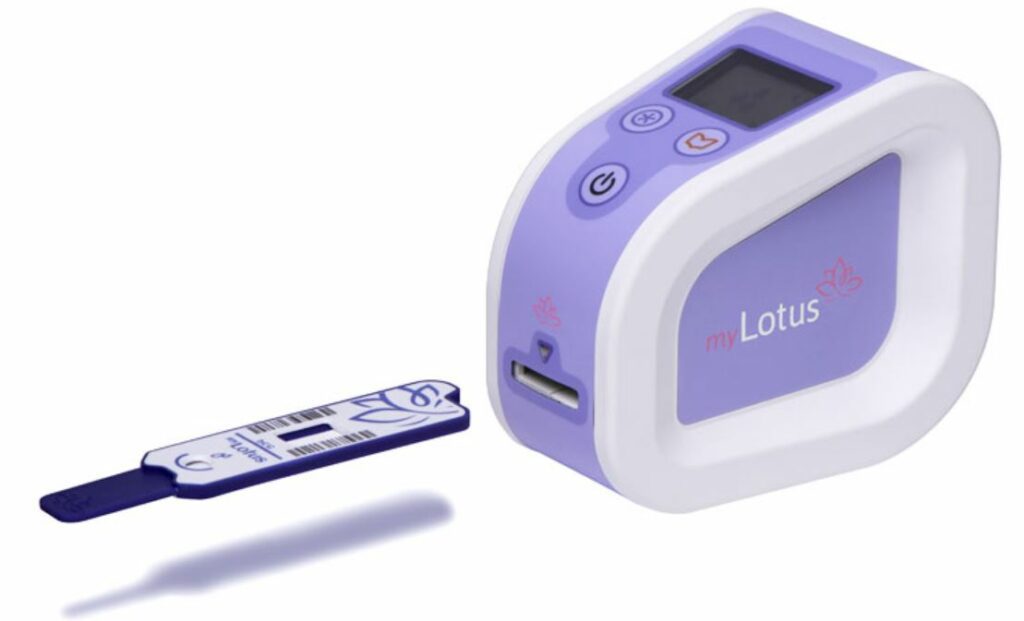
Read MoreIn-Home Fertility Tracking System Wins CE Mark
Concepta plc, the innovative UK healthcare company and developer of the proprietary self-test platform (“myLotus”) and suite of emerging test products targeting the mobile health market is pleased to announce it has received CE-Mark certification for its myLotus branded products for women’s fertility. Given the stringent regulatory environment for diagnostics, CE certification is a major milestone…
Read More