Archive for September 2019
Brainlab Introduces Mobile Intraoperative Imaging Robot
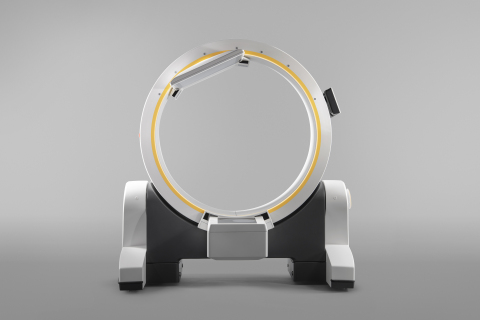
Brainlab, the digital medical technology company, unveiled Loop-X™, the first mobile intraoperative imaging robot today at NASS 2019 in Chicago. Loop-X sits at the core of the Brainlab Digital Surgery portfolio for the rapidly evolving spinal market. Brainlab is leveraging its long history in 3D imaging and open integration to bring to market a new benchmark…
Read MoreSamsung to Unveil New Premium Ultrasound System at Society of Diagnostic Medical Sonography Conference of 2019
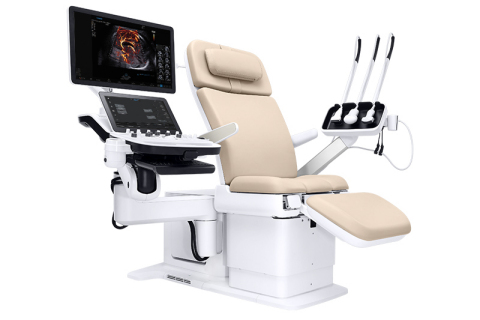
NeuroLogica, a subsidiary of Samsung Electronics Co. Ltd., announced that the Samsung Hera I10 ultrasound system has received FDA clearance and will be unveiled at the Society of Diagnostic Medical Sonography (SDMS) 2019 Annual Conference in National Harbor, MD. The Hera I10 is the latest addition to the Samsung Hera family of Premium Women’s Health ultrasound systems…
Read MoreFDA Grants Breakthrough Status to Aurora’s Concussion Treatment
There were 2.5 million emergency department visits related to traumatic brain injuries in the U.S. in 2014, according to the Centers for Disease Control and Prevention (CDC). Trends seen in the run up to 2014, the last year on which CDC has published data, suggest the figure may have risen since then. An earlier CDC report found mild traumatic…
Read MoreEthicon Launches Industry’s First Powered Circular Stapler
To help address a serious complication associated with colorectal, gastric and thoracic surgery, Johnson & Johnson Medical Devices Companies* announced today that Ethicon** has launched the industry’s first powered circular stapler. The ECHELON CIRCULAR Powered Stapler reduces leaks by 61% at the staple line compared to Medtronic’s DST Series™ EEA™ Stapler.1 The new ECHELON CIRCULAR…
Read MoreAmbulatory Surgery Center Growth Accelerates: Is Medtech Ready?
An increase in the number of surgical procedures taking place at ambulatory care centers could change the playing field for medtech companies, according to a new report by Bain & Co. The management consulting firm predicted that procedures at these stand-alone centers could grow by 6% to 7% annually through 2021, with orthopedic, spine and cardiac procedures…
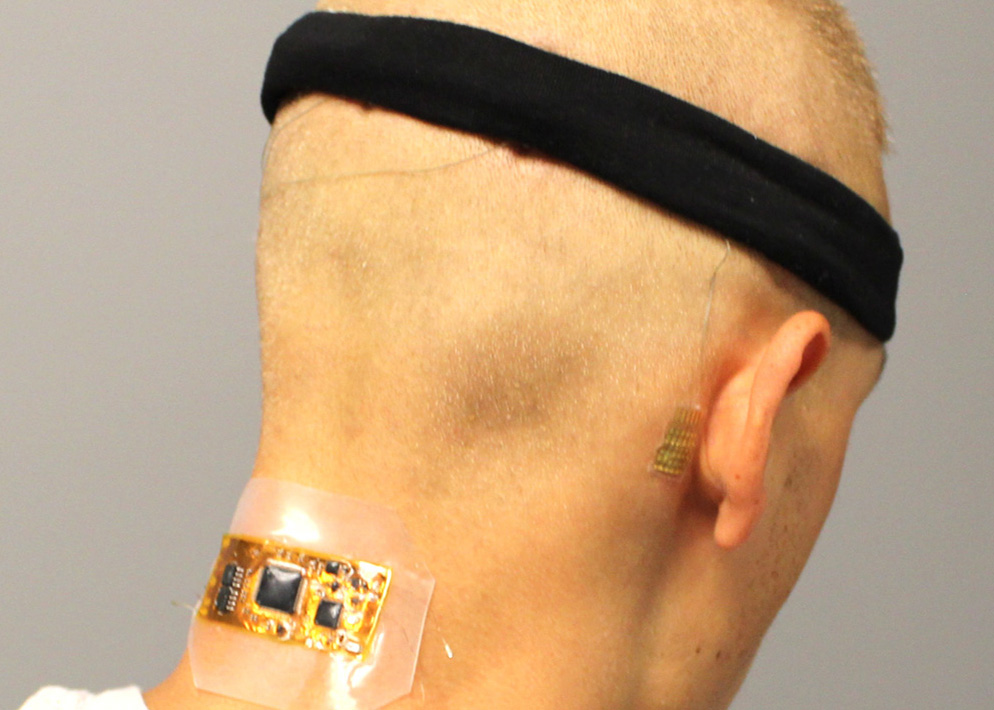
Read MoreFirst Portable Brain-Computer Interface to Control Wheelchairs, TVs, Computers
Brain-computer interfaces have the potential to give severely disabled people the ability to easily control their wheelchairs, televisions, and other devices. Existing technologies suffer from a number of limitations, though, making them impractical for real-world applications. One is that non-invasive brain wave monitoring currently requires large and uncomfortable electroencephalography caps with wet electrodes, wires, and…
Read MoreFDA Continues to Take Steps to Fulfill its Commitment to Strengthen and Modernize the 510(k) Medical Device Program
The U.S. Food and Drug Administration today announced that, as a first step toward implementation of the recently established Safety and Performance Based Pathway for medical devices, the agency is issuing draft guidances outlining the recommended premarket performance criteria and testing methodologies for four specific types of devices. These are anticipated to be the first device types…
Read MoreMedtronic Finally Unveils its New Robot-Assisted Surgery System
Medtronic has a robot-assisted surgery platform that is upgradeable, modular and able to perform both RAS and laparoscopic applications — an obvious answer to the da Vinci SP robot-assisted surgery platform made by Intuitive Surgical. That appears to be the message out today from the world’s largest medical device company as it unveils its much-awaited robot-assisted…
Read MoreCardiovascular Systems, Inc. Announces FDA Approval of the Coronary ViperWire Advance® With Flex Tip
Cardiovascular Systems, Inc. (CSI) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, today announced U.S. Food and Drug Administration (FDA) PMA approval of the new ViperWire Advance® Coronary Guide Wire with Flex Tip (ViperWire Advance with Flex Tip). ViperWire Advance with Flex Tip…
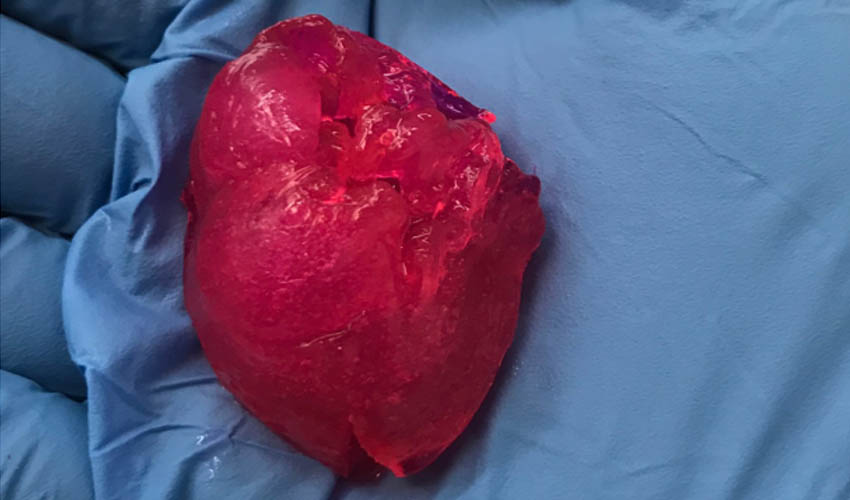
Read MoreBIOLIFE4D Bioprints Small Human Heart for the First Time in the U.S.
BIOLIFE4D, one of the pioneers in the bioprinting field, has been able to bioprint a miniature human heart – making it the first U.S. company to successfully achieve this. The company’s mission is to create a fully functioning human heart through bioprinting and using patient’s own cells in order to eliminate the challenges of organ rejection…
Read More