Neuroscience
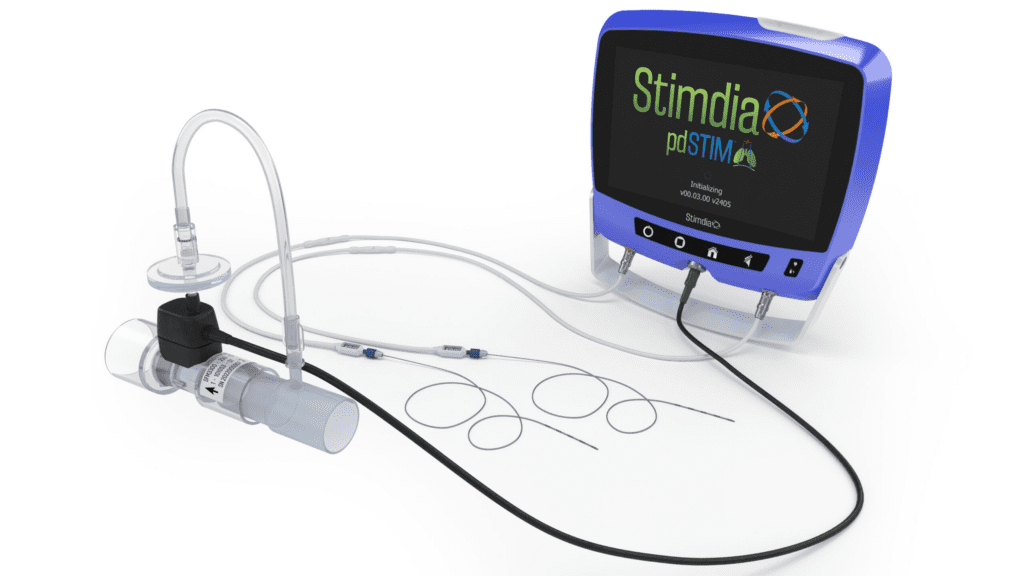
Stimdia Medical Inc. Initiates FDA Approved IDE Pivotal Trial to Investigate Impact of Neuromuscular Stimulation on Mechanically Ventilated Patients
Stimdia Medical, a medical device company developing innovative technologies to improve respiratory care, today announced the initiation of its FDA approved pivotal trial to investigate the impact of phrenic nerve stimulation (PNS) on the time to liberate patients from mechanical ventilation. The ReInvigorate Study is a randomized, controlled trial that will enroll approximately 420 patients…
Read MoreCereVasc Receives FDA IDE Approval to Expand its Clinical Study of Patients with Normal Pressure Hydrocephalus Utilizing the Generation 2 eShunt® System
CereVasc, Inc., a clinical-stage, medical device company developing novel treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) supplement to permit the expansion of the study of the eShunt System in patients with Normal Pressure Hydrocephalus (NPH) to additional study participants and clinical sites.…
Read MoreMedRhythms Announces FDA Listing of InTandem™ (MR-001) to Improve Walking and Ambulation in Adults with Chronic Stroke
MedRhythms announced that MR-001, the company’s evidence-based neurorehabilitation system to improve walking and ambulation in adults with chronic stroke walking deficits, has been listed as a Class II medical device with the U.S. Food and Drug Administration (FDA). The system delivers an intervention based on the principle of Rhythmic Auditory Stimulation (RAS), a well-researched clinical…
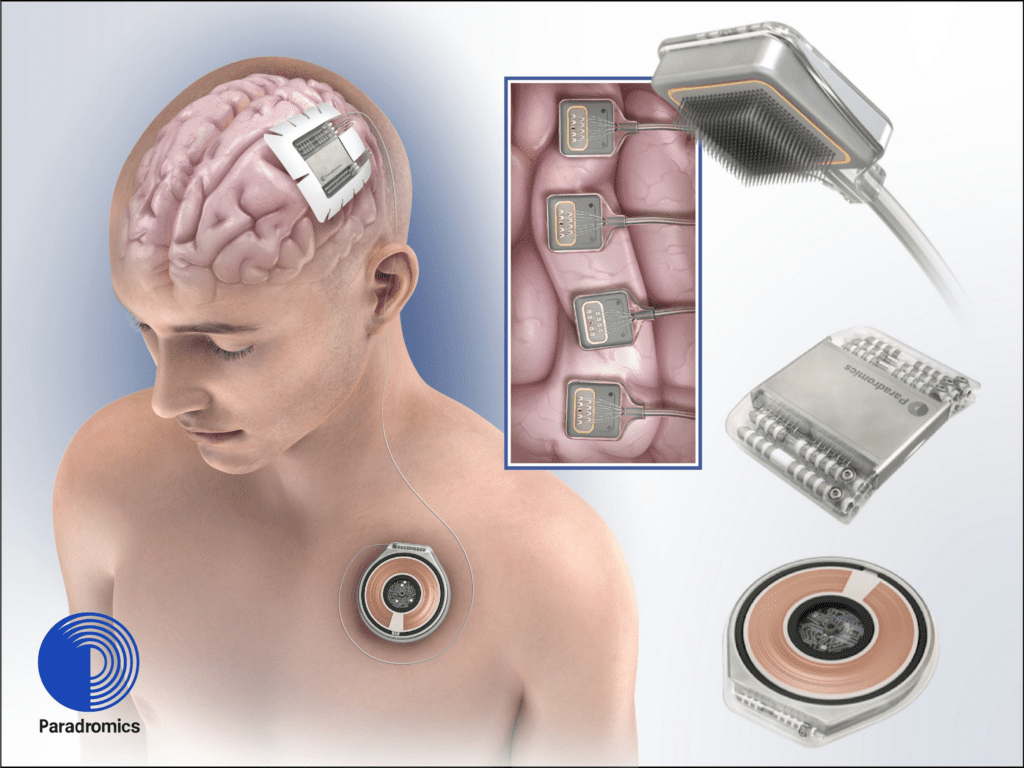
Read MoreParadromics Raises $33 Million in Funding, Achieves Breakthrough Medical Device Designation from FDA
Paradromics Inc., the leading developer of high data-rate brain-computer interfaces (BCI), today announced a $33 million Series A funding round led by Prime Movers Lab. Additional investors include Westcott Investment Group, Dolby Family Ventures, and Green Sands Equity. The new funding will help Paradromics launch its first-in-human clinical trial for the Connexus® Direct Data Interface (DDI). In addition…
Read MoreNeuvotion Secures an Additional $1.25M to Commercialize NeuStim™ Technology for Treating Stroke and Debilitating Injuries
Neuvotion, Inc. is an early-stage medical device company developing neurostimulation products for the rehabilitation, brain-computer interface, and physical therapy markets, today announced that it has received an additional $1.25M in funding, bringing cumulative funding to over $2.75M. Northwell Holdings, the venture investment arm of Northwell Health, Topspin Fund, and Long Island Angel Network also participated in the round. This…
Read MoreSynapse Biomedical Wins New Approval for Diaphragm Pacing System to Free Patients from Ventilators
Synapse Biomedical, Inc. announced today that the FDA has granted premarket approval (PMA) of the NeuRx® Diaphragm Pacing System (NeuRx DPS®) for use in patients with spinal cord injuries who rely on mechanical ventilation. PMA is the most stringent type of device marketing application required by FDA. More hospitals are expected to begin implementing the NeuRx…
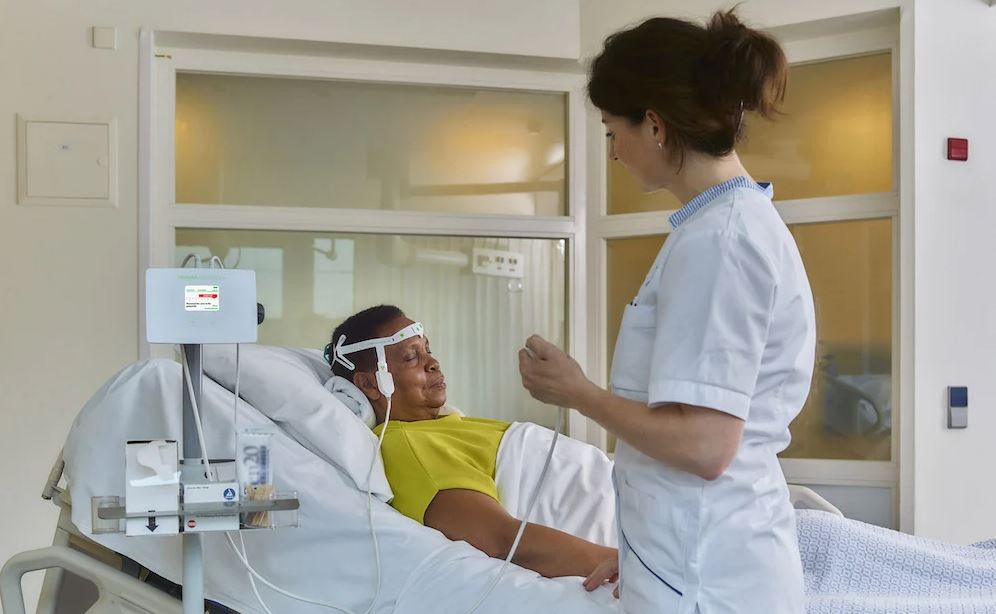
Read MoreProlira Announces FDA Clearance of the DeltaScan® Brain State Monitor for Detecting Acute Encephalopathy (Acute Brain Failure), a Condition Affecting Millions of Hospitalized Patients 60 and Older
Prolira BV, a high-tech company committed to enabling early and effective treatment of patients at risk of developing acute brain failure, announced it has received 510(k) clearance from the Food and Drug Administration (FDA) for the DeltaScan® Brain State Monitor to aid in the diagnosis of acute encephalopathy in hospitalized patients over 60 years of…
Read MoreAxonics Receives FDA Approval for Fourth-Generation Rechargeable Sacral Neuromodulation System
Axonics, Inc., a global medical technology company that is developing and commercializing novel products for the treatment of bladder and bowel dysfunction, announced that the U.S. Food and Drug Administration has approved the company’s fourth-generation rechargeable sacral neuromodulation system. The newly approved Axonics R20™ neurostimulator is labeled for a functional life in the body of at…
Read MoreSonorous NV Uses New Device to Treat Patients with Symptomatic Cerebral Venous Diseases
Sonorous NV, Inc. Chairman, Dave Ferrera, announced treatment of the first patients in North America with the BossStent® device designed to treat patients with symptomatic cerebral venous diseases. The single-use, implantable device acts as an endoluminal prothesis that is placed into a cerebral venous sinus stenosis to normalize blood flow and pressure gradients causing pulsatile tinnitus and/or IIH (idiopathic intracranial hypertension),…
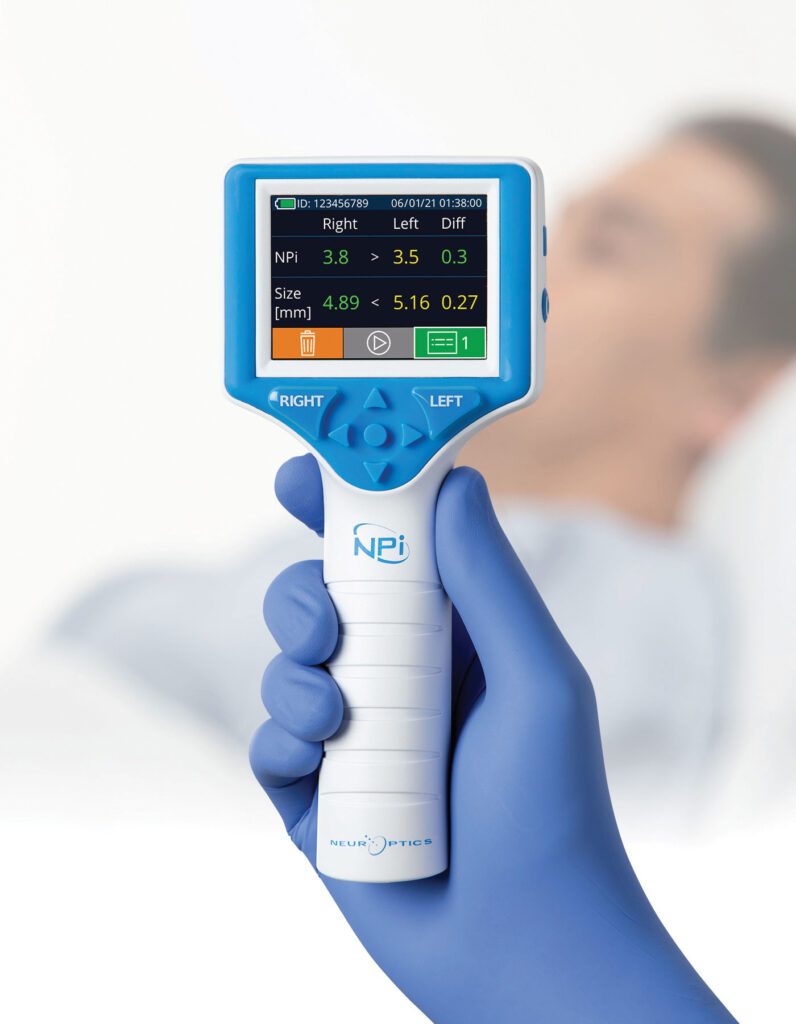
Read MoreNeurOptics’ Neurological Pupil index™ and Automated Pupillometry Included in Latest European Guidelines for Post-Resuscitation Care
NeurOptics’ Neurological Pupil index™ (NPi®) and automated pupillometry are now included in the updated 2021 European Resuscitation Council (ERC) and European Society of Intensive Care Medicine (ESICM) Guidelines for post-resuscitation care as an objective measurement supporting neurological prognosis in patients following cardiac arrest. The ERC and ESICM guidelines outline the latest European post-resuscitation care science…
Read More