Archive for May 2022
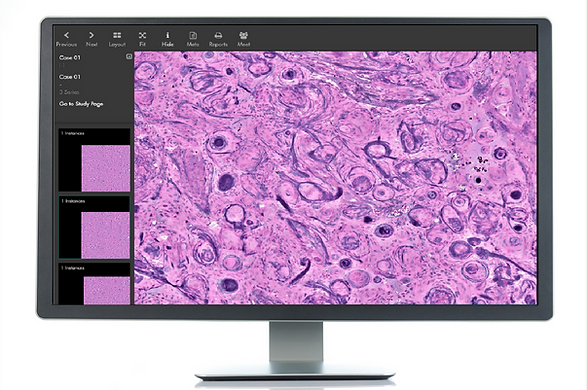
Invenio Imaging Receives CE Mark to Detect Cancer at the Time of Surgery using Artificial Intelligence
Invenio Imaging, the leader in intraoperative fresh tissue imaging, announced the CE Mark for the NIO Glioma Reveal image analysis module. NIO Glioma Reveal is based on deep learning and allows neurosurgeons to identify areas of cancer infiltration in patients undergoing primary treatment of a diffuse glioma. With the CE mark, neurosurgeons in the European…
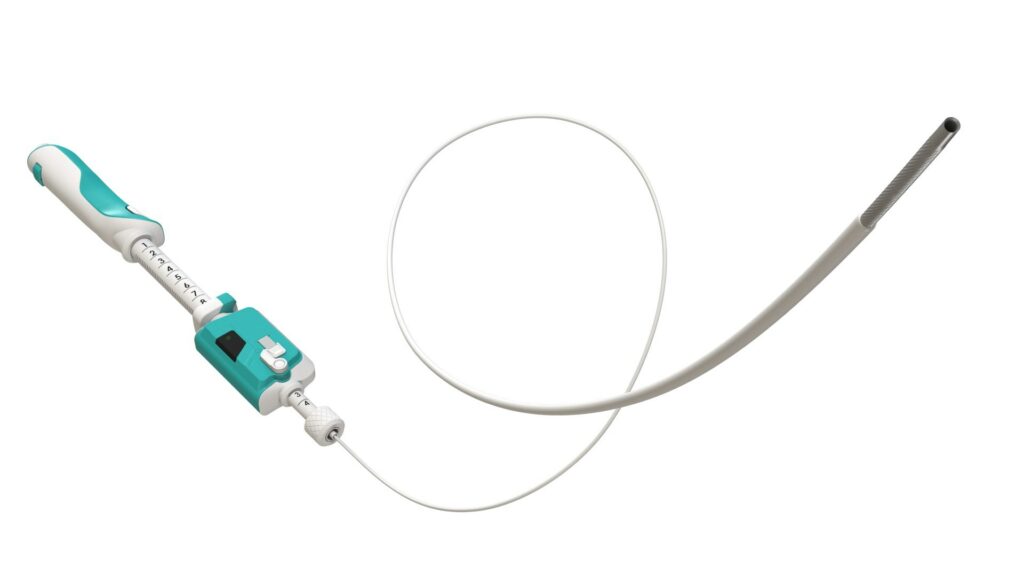
Read MoreLumendi Files for FDA 510(k) Clearance for Two New Products
Lumendi, LLC has announced it has filed for U.S. Food and Drug Administration (FDA) 510(k) clearance for DiLumen EZ¹, a single-use, disposable endotherapy device intended for endoscopic mucosal resections (EMR) and difficult colonoscopies. The new design, based on the long-standing success of the DiLumen EZ-Glide platform, has been modified and streamlined. “This new device will…
Read More5 Ways To Become Your Recruiter’s Favorite Candidate
When many people start to work with a recruiter, they have no idea what our jobs really entail. Lots of candidates like to assume that we only work on one position opening at a time, talk to a few select candidates, and then place one. This often leads well-meaning candidates to do things like leave…
Read MoreArticle Reviews the Evolution of Arthroscopy from its Invention in 1912 to the Dawn of the Wireless ArthroFree™ Era
A recent history of arthroscopy by Dr. James S. Williams, Jr., and Dr. Asheesh Gupta reports that while there have been numerous incremental advances in arthroscopic camera technology over the last half-century, the current systems remain largely unchanged. Surgeons are tethered to the surgical tower by a power cable and by a light cord that…
Read MoreiCAD’s 3D Mammography AI Improves Cancer Detection Accuracy
iCAD, Inc., a global medical technology leader providing innovative cancer detection and therapy solutions, announced that new research supporting ProFound AI® for Digital Breast Tomosynthesis (DBT) will be presented at the Society of Breast Imaging (SBI/ACR) Breast Imaging Symposium, taking place May 16-19 in Savannah, GA. The Company is also showcasing its complete suite of deep-learning breast…
Read MoreOur Interview Secret Weapon: The 30-60-90 Day Plan!
You have a great resume, you talked to the right people, and you got your foot in the door; you got to the interview stage of your job hunt. However, so did five other people, but there is something you can do to stand apart from your competition: bring a 30-60-90 day plan to your…
Read MoreExactech Announces FDA Breakthrough Device Designation for JointMedica’s Polymotion® Hip Resurfacing System
Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced that the U.S. Food and Drug Administration (FDA) has granted a Breakthrough Device Designation for JointMedica’s Polymotion® Hip Resurfacing System. Exactech, a minority shareholder of JointMedica Limited, is collaborating with the United Kingdom-based orthopaedic device designer and manufacturer to deliver…
Read MoreLimaca Medical Receives FDA Breakthrough Device Designation
Limaca Medical (“Limaca”) announced that its Precision-GI™ Endoscopic Ultrasound Biopsy Product has received a Breakthrough Device Designation from the U.S. Food and Drug Administration (“FDA”). The Precision-GI™ device is designed to obtain tumor tissue within or adjacent to the gastrointestinal tract. Endoscopic biopsy is performed by a gastroenterologist who accesses the targeted GI tumor utilizing an…
Read MoreNetwork to Sharpen your Long Term Job Search Strategy
The reality of today is there is seldom a permanent job: With ever-growing competition, and the increased frequency of mergers, acquisitions, closures, offshoring, etc. have demonstrated that even the most tenured employee does not have long-term job certainty. All of these factors affect employees regardless of seniority, management/staff level, function or industry. Take the necessary…
Read MoreX-Therma Receives FDA Breakthrough Device Designation for XT-ViVo® Preservation Solution and TimeSeal® Organ Transport Device
X-Therma Inc., a biotechnology company developing breakthrough technology for regenerative medicine and organ preservation – announced today that The Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) has granted its proprietary organ preservation solution, XT-ViVo®, and TimeSeal® Organ Transport Device, Breakthrough Device status. This designation is granted to products that have…
Read More