Archive for March 2022
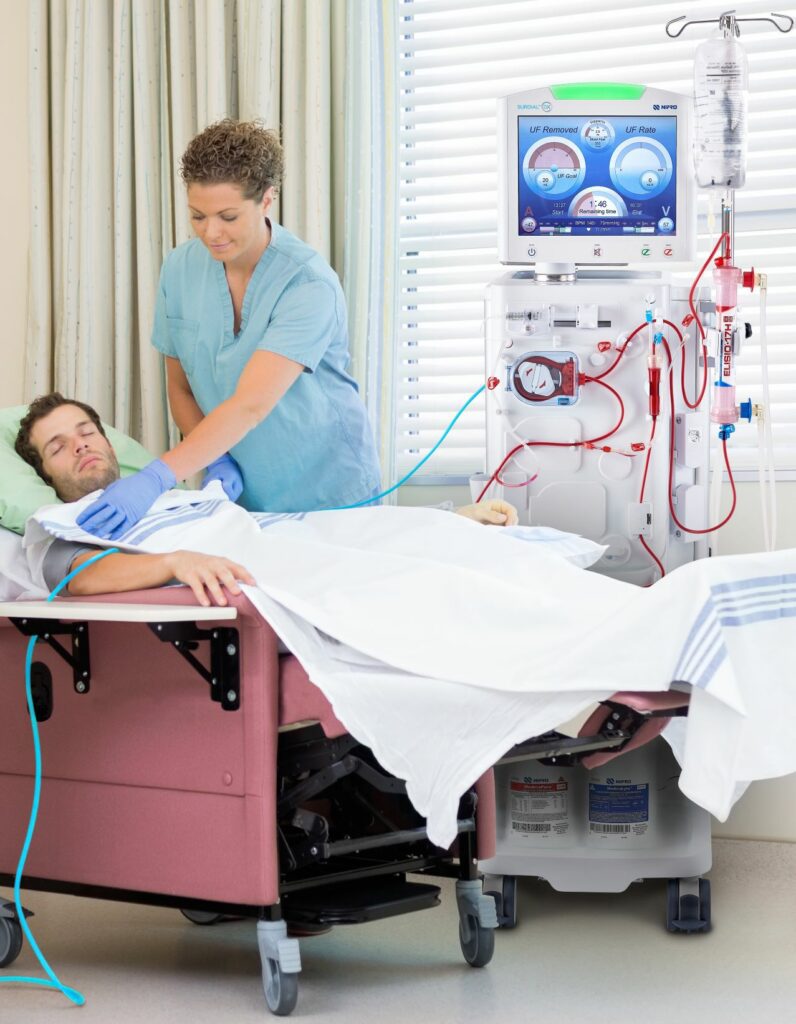
Nipro introduces SURDIAL™ DX Hemodialysis System to U.S. Market
Nipro Medical Corporation (Nipro), a leading manufacturer and supplier of renal, vascular, and medical-surgical products, announced the commercial launch of SURDIAL™ DX Hemodialysis System to the U.S. SURDIAL™ DX is a state-of-the-art hemodialysis system designed to create an optimal dialysis treatment experience for patients and clinicians. Manufactured in Japan, it draws from over 35 years of…
Read MoreTendoNova wins FDA Clearance for Its New Microinvasive Ocelot Surgical Tool
TendoNova, an emerging leader in microinvasive sports medicine procedures, is pleased to announce the FDA 510(k) clearance of its new Ocelot surgical tool, which uses an innovative technology for fragmentation or debridement of soft tissue. The FDA found the Ocelot TDS 1000 substantially equivalent to the Tenex TX1 (recently acquired by Trice Medical) which is…
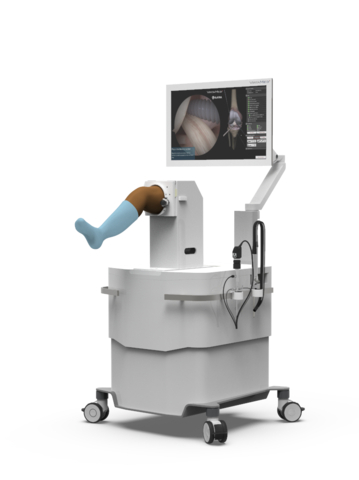
Read MoreVirtaMed Announces New Inclusive Knee Simulation Model
VirtaMed, the world leader in medical simulation training, has announced the release of enhanced simulation training modules for arthroscopic knee surgeries. In a first for VirtaMed, these latest training modules will now be available on simulators with two different skin tones. “It is important to learn medical skills in a realistic environment, and that means…
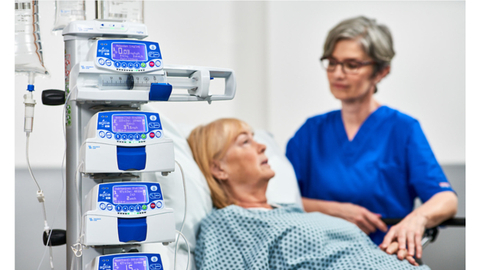
Read MoreFresenius Kabi Receives FDA 510(k) Clearance for Wireless Agilia® Connect Infusion System with Vigilant® Software Suite-Vigilant® Master Med Technology
Fresenius Kabi, a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition, announced it has received 510(k) regulatory clearance from the U.S. Food and Drug Administration (FDA) for its wireless Agilia® Connect Infusion System which includes the Agilia® Volumetric Pump and the Agilia® Syringe Pump with Vigilant® Software Suite-Vigilant® Master Med technology. The Agilia Connect…
Read MoreCoapTech to Initiate Clinical Trial for its PUMA-G Peds System for Pediatric Gastrostomy
CoapTech, Inc, a medical device company focused on delivering transformative solutions for minimally-invasive surgery, announced it has received an Investigational Device Exemption (IDE) approval from the Food and Drug Administration (FDA) to initiate a clinical trial for its PUMA-G Peds System, a device designed to provide a safer and more efficient way to place feeding tubes in…
Read MoreEchosens Launches FibroScan GO, Affordable State-of-the-Art, Non-Invasive Solution for Identifying Patients with Advanced Chronic Liver Disease
Echosens, a high-technology company offering the FibroScan® portfolio of solutions, is pleased to announce the launch of FibroScan GO, an affordable, cost-effective tool for screening liver health, improving liver health management at the point of care for primary care practices and reducing the flow of patients in secondary care centers. FibroScan GO is a solution for…
Read MoreLazurite™ to Showcase its ArthroFree™ Wireless Camera System at Multiple Upcoming Conferences
Medical device and technology company Lazurite Holdings LLC announced that its ArthroFree™ wireless camera system for minimally invasive surgery will be available for viewing and hands-on demonstration at these four upcoming medical conferences: Annual Meeting of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), March 16-19, in Denver (Booth 202) Global Surgical Conference & Expo…
Read MoreValencia Technologies Gains FDA Approval of eCoin® Therapy to Treat Urinary Urge Incontinence
Valencia Technologies Corporation, a privately held company, announced that the U.S. Food and Drug Administration (FDA) has granted premarket approval (PMA) of its eCoin® leadless tibial neurostimulator for the treatment of urinary urge incontinence (UUI), which affects over 60% of patients who suffer from Overactive Bladder (OAB). FDA approval was supported by the efficacy and favorable…
Read More