Archive for June 2020
EO and UVDI Lead Trans-Pacific Fight to Flatten the COVID-19 Curve
EO Medical Pte. Ltd., announced in partnership with UltraViolet Devices, the global leader in UV-C disinfecting robots, that six additional UVDI-360 robots have been deployed in Singapore to curb the spread of coronavirus. UVDI-360 UV-C robots, manufactured in the greater Los Angeles area, are the most widely used disinfecting robot in Singapore’s top hospitals. “Protecting…
Read MoreLeMaitre Vascular Buys Artegraft for $90M
LeMaitre Vascular, Inc. announced that it has acquired the business and assets of Artegraft, Inc. for $90.0 million, including $72.5 million in cash paid at closing ($65.0 million to Artegraft plus $7.5 in escrow to be released December 31, 2021) as well as potential earnout payments of $17.5 million payable based upon future sales of…
Read MoreBreakthrough Device Designation Given for preCARDIA’s Catheter Based Heart Failure Treatment
preCARDIA, Inc., has announced that the company’s catheter based system for treating volume overload in patients with acutely decompensated heart failure (ADHF) has been designated for the Breakthrough Devices Program by the U.S. Food and Drug Administration (FDA). The FDA’s Breakthrough Device Program was established for medical technologies that have the potential to provide more…
Read MoreFirst US Patients Treated Using the CardioFocus HeartLight X3 Ablation System
CardioFocus, Inc. today announced that the first U.S. patients have been treated commercially with the recently-approved HeartLight® X3 Endoscopic Ablation System. The revolutionary cardiac ablation technology is designed to treat drug refractory, symptomatic paroxysmal atrial fibrillation (AFib), the most common heart rhythm disorder. Henry D. Huang, M.D., FACC, FHRS of Rush University Medical Center in Chicago, and David Kenigsberg M.D., FACC, FHRS…
Read MoreNeovasc Closes $11.5 Million Offering
Neovasc Inc. (“Neovasc” or the “Company”), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies, and minimally invasive devices for the treatment of refractory angina, announced today that it has closed its previously announced registered direct offering (the “Offering”) priced at-the-market under Nasdaq rules of an aggregate of 3,883,036 units (the…
Read MoreLifeScan Announces OneTouch Verio Reflect Meter U.S. Launch
LifeScan, a world leader in blood glucose monitoring and maker of the iconic OneTouch® brand, today announced the U.S. launch of OneTouch Verio Reflect®, the only meter with a Blood Sugar Mentor™ feature that gives people with diabetes personalized real-time guidance to help them take action to maintain and/or improve control. The OneTouch Verio Reflect meter…
Read MoreShockwave Medical Announces That CMS Has Created New Codes for Intravascular Lithotripsy
Shockwave Medical, Inc., a pioneer in the development and commercialization of Intravascular Lithotripsy (IVL) to treat complex calcified cardiovascular disease, announced today that the Centers for Medicare & Medicaid Services (CMS) has issued new codes for IVL procedures performed in peripheral arteries in both the hospital outpatient and inpatient settings. The new Healthcare Common Procedure…
Read More4WEB Medical Announces FDA 510(k) Clearance of Stand-Alone Anterior Lumbar Interbody Fusion Device
4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design that utilizes its proprietary Truss Implant Technology™, announced today that the company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Stand-Alone Anterior Lumbar Interbody Fusion Device (ASTS-SA). The new design allows fixation screws to…
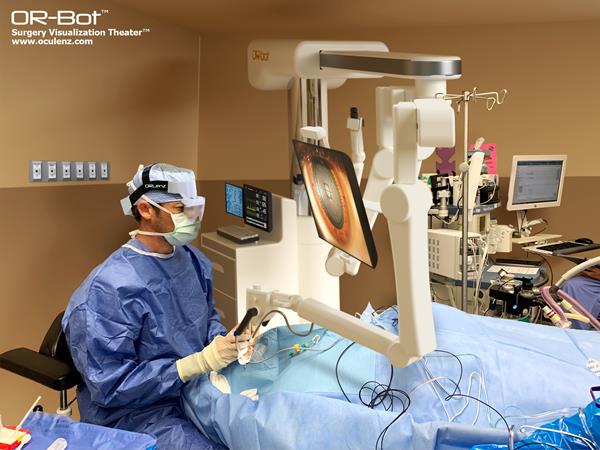
Read MoreOcutrx’s Oculenz Advances from AR Headset to Full Surgery Visualization Theatre
The maker of breakthrough augmented-extended reality (AR/XR) glasses that provide a radically new surgical viewing experience for retinal surgeons and patients alike, is launching new technology that provides the most modern options for surgery visualization and to remove “pain” for surgeons to improve surgeries, Ocutrx Vision Technologies, LLC announced today. The Ocutrx OR-Bot™️ Surgery Visualization Theatre™ will…
Read MoreCMS Approves SINUVA Sinus Implant for Reimbursement with New C-Code and Pass-Through Payment Status
Intersect ENT, Inc., a company dedicated to transforming care for patients with ear, nose and throat conditions, today announced that the Centers for Medicare and Medicaid Services (CMS) has approved SINUVA® (mometasone furoate) Sinus Implant for transitional pass-through payment status for reimbursement under the Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center Payment…
Read More