Archive for June 2020
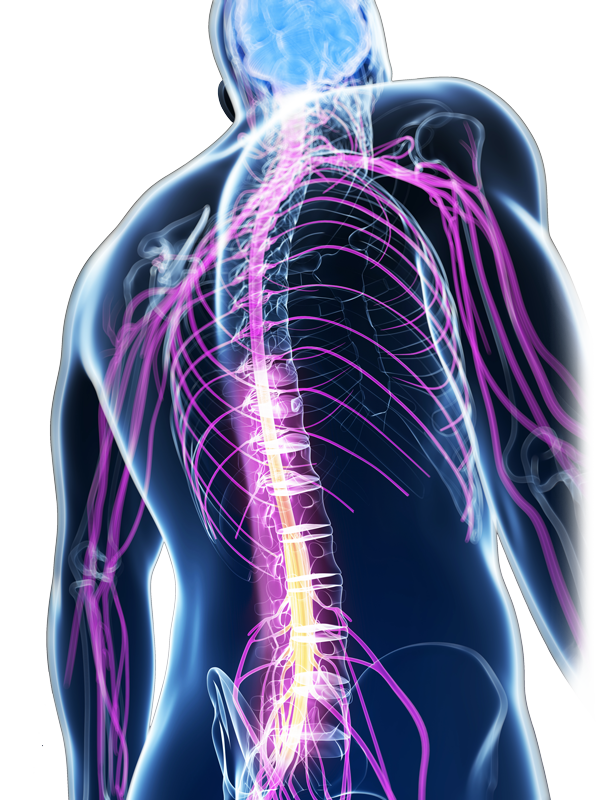
GTX medical Granted FDA Breakthrough Device Designation for Go-2 Targeted Epidural Spinal Stimulation (TESS) System
GTX medical (GTX), today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its implantable Go-2 system which was designed to promote the recovery of leg motor functions and neurological control in adults with spinal cord injuries (SCI) and paralysis. The Go-2 System provides Targeted Epidural Spinal Stimulation (TESS)…
Read MoreNuvaira Announces FDA Breakthrough Designation for AIRFLOW-3 Pivotal Trial Device
Nuvaira, a developer of novel therapeutic strategies to treat obstructive lung diseases, has announced that its Nuvaira® Lung Denervation System has been designated as a Breakthrough Device by the U.S. Food and Drug Administration (FDA). This program creates an expedited pathway for prioritized FDA review of devices that have potential to provide more effective treatment…
Read MoreVasQ External Support Awarded Breakthrough Device Designation by the FDA
The FDA has designated Laminate Medical’s VasQ™ External Support for the creation of arteriovenous fistulas (AVF) in hemodialysis patients as a Breakthrough Device. The FDA Breakthrough Device Program is intended to provide patients and doctors timely access to medical devices that are more effective than the current standard of care for life-threatening or irreversibly debilitating…
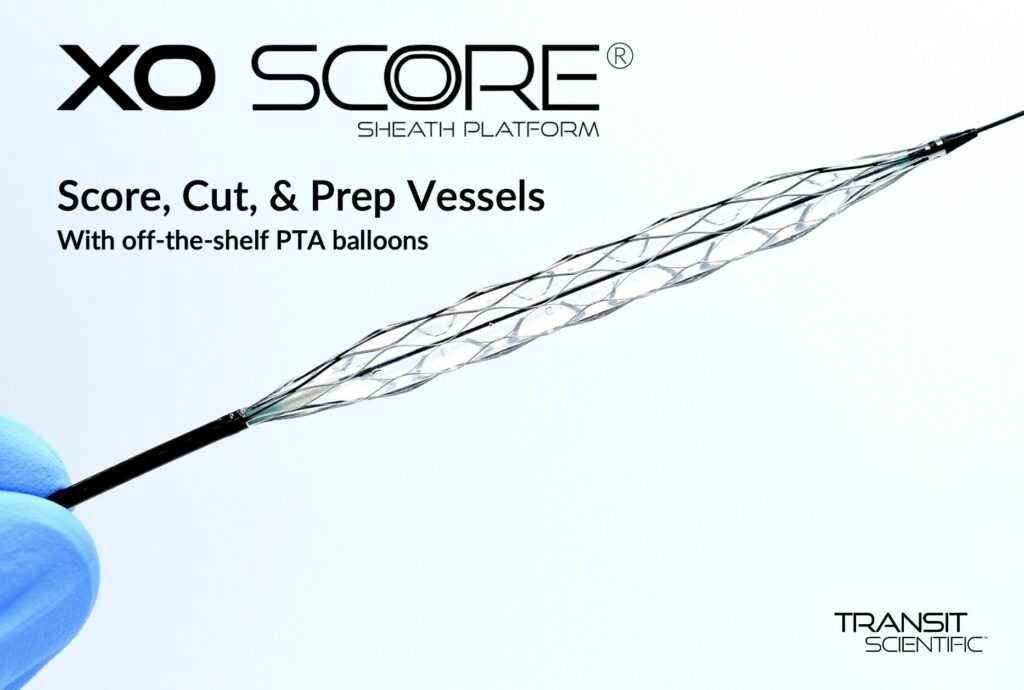
Read MoreFDA Clears Innovative Angioplasty Scoring and Cutting Platform
Transit Scientific announced the FDA cleared the XO Score® Percutaneous Transluminal Angioplasty (PTA) Scoring Sheath platform for use in iliac, ilio-femoral, popliteal, infra-popliteal, and renal arterial plus synthetic and/or native arteriovenous hemodialysis fistula. Angioplasty is performed with expandable polymer balloon catheters to dilate stenosed, or narrowed, vessels. Calcified, fibrous, and/or resilient stenosis may require special scoring…
Read MoreTitan Medical Announces Development and License Agreements With Medtronic and Senior Secured Loan
Titan Medical Inc. (“Titan” or “Titan Medical”) (TSX: TMD) (Nasdaq: TMDI), a medical device company focused on the design and development of single-port robotic surgical technologies, announces that it has entered into a development and license agreement with Medtronic plc (“Medtronic”) (NYSE: MDT) to further the development of robotic assisted surgical technologies, as well as…
Read MoreNoctrix Health granted FDA Breakthrough Device Designation for wearable Restless Legs Syndrome Therapy
Noctrix Health, Inc., a privately held medical device company announced today that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its wearable NTX100 Neuromodulation therapy designed for the treatment of adults with primary moderate-severe Restless Legs Syndrome (RLS) who are refractory to medications. RLS is the second most common sleep disorder in…
Read MoreAesculap Inc. Announces U.S. Launch of M.blue Hydrocephalus Valve
Aesculap Inc., in partnership with The Christoph Miethke GmbH & Co. KG (MIETHKE), is pleased to announce the launch of the M.blue valve, the latest generation of Hydrocephalus valve technology. Its unique gravitational technology is integrated with a fixed differential pressure unit in one valve, allowing for a simple, position-dependent solution. The M.blue valve is…
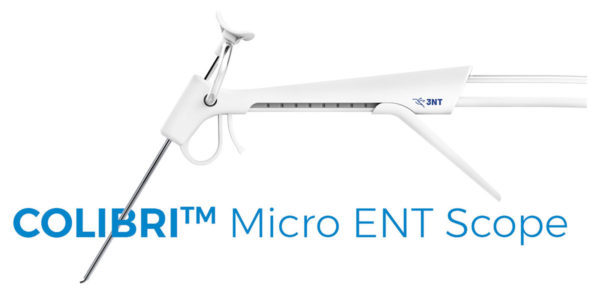
Read More3NT Medical Announces FDA Clearance for Colibri Endoscopy System
3NT Medical, a privately-held corporation dedicated to developing single-use specialized endoscopes for the diagnosis and treatment of ear, nose and throat (ENT) disorders, announced today the FDA 510(k) clearance for the Colibri™ Micro ENT Scope, the world’s first single-use endoscope specifically designed for Otology. The Colibri™ endoscopy system incorporates a lightweight ergonomic handpiece, a 2.2mm…
Read MoreArterys Raises $28 Million to Accelerate the Delivery of Medical AI to Practices Around the World
Arterys, a leading global medical imaging platform to deliver clinical AI products over the internet, just received its seventh USA FDA clearance and announced its latest round of funding — a $28 million Series C investment from a syndicate led by Benslie Investment Group and Temasek Holdings, with participation by Fosun, Revelation Partners, Emergent Medical…
Read MoreFDA Issues Emergency Use Authorization for Impella RP as Therapy for COVID-19 Patients with Right Heart Failure
The United States Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Impella RP to include patients suffering from COVID-19 related right heart failure or decompensation, including pulmonary embolism (PE). Abiomed (NASDAQ: ABMD) manufactures Impella RP. Impella RP is a temporary heart pump that provides circulatory support for patients who develop right side ventricular failure.…
Read More