Archive for July 2019
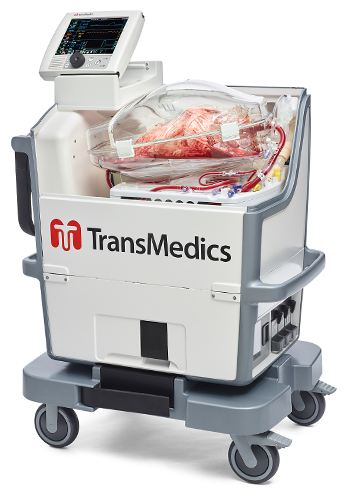
TransMedics Touts Long-Distance Organ Transport from Hawaii to North Carolina
TransMedics Group, Inc., a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart and liver failure, today announced the OCS Lung technology was used in two successful lung transplants using donor lungs from Hawaii that initially were declined for transplantation due to time and distance limitations of standard cold…
Read MoreAllergan Recalls Textured Breast Implants Linked to Rare Type of Cancer
The FDA said today that it asked Allergan to recall its Biocell breast implants after concluding that they are six times as likely as other textured breast implants to cause an uncommon form of lymphoma. The federal safety watchdog said that nearly 84% of the 573 cases of breast implant-associated anaplastic large cell lymphoma reported worldwide…
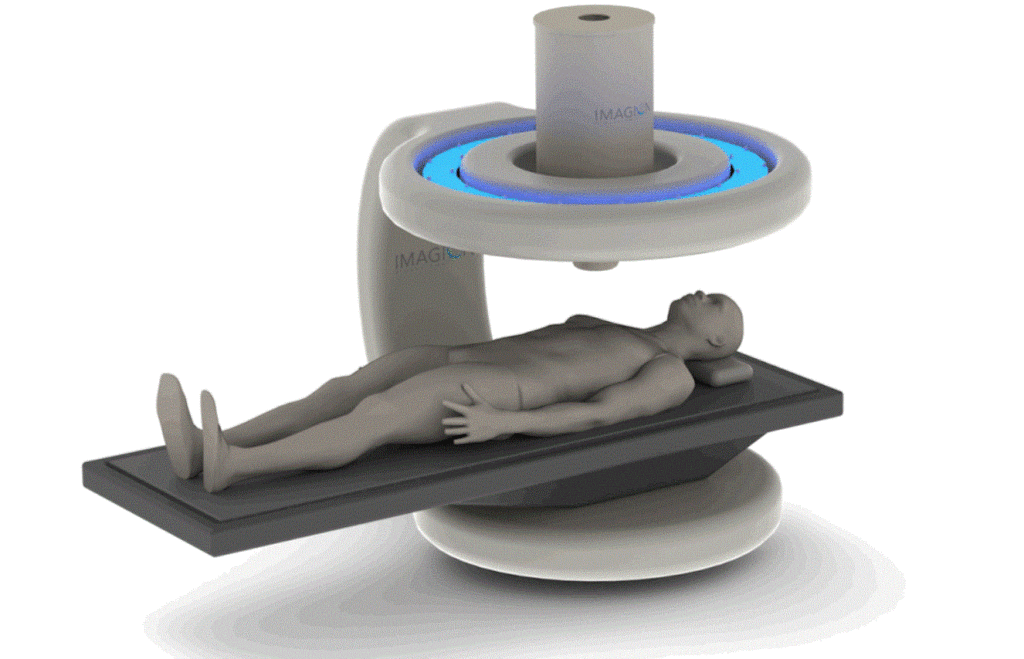
Read MoreImagion Biosystems Receives FDA Breakthrough Device Designation
Imagion Biosystems is working to differentiate itself in cancer detection with a system that aims to minimize the need for surgical or biopsy procedures to obtain tissue for pathological assessment. It says the technology as an alternative to MRI, PET, CT and X-ray for faster detection and treatment than conventional imaging methods, according to a presentation to…
Read MoreMedtronic to Distribute Viz.ai’s Stroke Imaging Software
Medtronic, a global leader in medical technology, and Viz.ai, the emerging leader in applied artificial intelligence (AI) in stroke care, have partnered to accelerate the adoption of Viz.ai’s new technology, which helps synchronize stroke care and decrease time to treatment, potentially improving outcomes for patients. Viz.ai’s technology uses artificial intelligence to identify suspected large vessel…
Read MoreCentinel Spine Announces IDE Approval of Two Different Prodisc C Devices
Centinel Spine said earlier this week that it won an investigational device exemption from the FDA for a clinical trial of its Prodisc cervical disc implants. New York City-based Centinel acquired the Prodisc assets from Johnson & Johnson subsidiary DePuy Synthes in December 2017 for an undisclosed amount. The FDA IDE covers a two-level study of the company…
Read MoreInsightec Receives FDA Approval and CE Mark for Exablate Neuro with GE SIGNA Premier MRI
GE Healthcare, a leader in medical technology and diagnostic and therapeutic imaging, and INSIGHTEC®, a global medical technology innovator of incisionless surgery, today announced FDA approval and CE mark for Exablate Neuro™ compatible with the SIGNA™ Premier MRI system from GE Healthcare. The Exablate Neuro is a focused ultrasound platform for treating deep in the…
Read MoreCenterline Biomedical Receives FDA 510(k) Clearance for Intra-Operative Positioning System
Centerline Biomedical, Inc. (Centerline) has announced that the company has received 510(k) clearance from the United States Food and Drug Administration (FDA) to market its flagship product, the Intra-Operative Positioning System, IOPS. A non-radiation-based surgical navigation system for minimally invasive surgery, IOPS leverages anatomical mapping algorithms and electromagnetic tracking technology to provide three-dimensional color visualization…
Read MoreThese Medical Devices are now Exempt from the 25% China Tariffs
On Tuesday, the U.S. exempted several categories of medical devices from the 25% tariffs imposed on Chinese goods by the Trump administration, including surgical, radiotherapy and dental devices. The Office of the U.S. Trade Representative said the new exclusions are retroactive to July 6, 2018, when tariffs went into effect on some $34 billion in imports…
Read MoreMediCool Technologies Welcomes New CEO to Lead Development of Novel AFib Therapy
Electrophysiology Industry Veteran to Lead Early Stage Commercialization MediCool Technologies, an early stage medical device company developing a novel device to painlessly terminate cardiac arrhythmias, today announced that the Company has appointed Mr. Jeff Rynbrandt as President and Chief Executive Officer. Mr. Rynbrandt brings to the Company 25 year’s leadership across multiple disciplines in both…
Read MoreCritical Innovations Receives Breakthrough Device Designation for FOAM Device
Critical Innovations announced earlier this week that the FDA has granted breakthrough device designation for its “Fast Onset Abdominal Management™ (F.O.A.M.™) device, which is designed to deliver a quickly-expanding foam to tamponade severe internal bleeding in trauma patients. F.O.A.M.™ research and development is supported by funding from U.S. Army Medical Materiel Development Activity (USAMMDA), through…
Read More