Archive for July 2022
Rapid Medical Gains FDA Clearance for the Smallest & Only Adjustable Thrombectomy Device
Rapid Medical, a leading developer of advanced neurovascular devices, announces FDA 510(k) clearance for TIGERTRIEVER™13 for large vessel occlusions at the 2022 Society of NeuroInterventional Surgery’s (SNIS) 19th Annual Meeting in Toronto. TIGERTRIEVER 13 is the smallest revascularization device in the world to date and is designed to remove thrombus from delicate brain blood vessels during an…
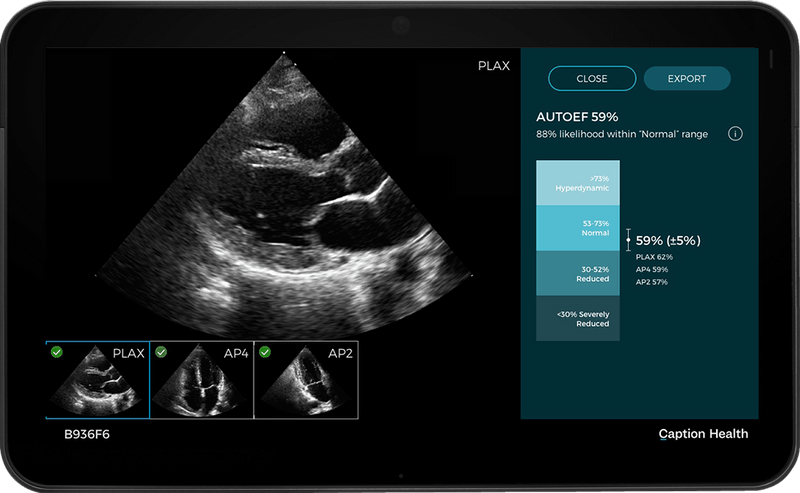
Read MoreCaption Health Receives CE Mark for Caption AI™ Technology Platform
Caption Health, the leader in using AI and services to improve heart ultrasound access, announced that it has received a CE Mark for its Caption AI™ technology platform. This certification represents the first step in making Caption Health’s industry-leading technology platform available outside the US, and highlights the company’s strong clinical and regulatory track record…
Read MoreHow and Why Your Resume Should be for TWO different audiences: Man and Machine
Resume writing advice is everywhere online. There are guides that proudly proclaim how important resume titles are, or how your Times New Roman font is preventing you from finding your next job, to guides that insist you use a professional service to “set yours apart from the rest.” The popular conception is that there’s stack…
Read MoreAncora Heart Receives Breakthrough Device Designation from FDA for the AccuCinch® Ventricular Restoration System
Ancora Heart, Inc., a company developing a novel device-based therapy to address heart failure, announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to the AccuCinch® Ventricular Restoration System. Currently being evaluated in the CORCINCH-HF pivotal clinical trial, the AccuCinch System is designed to provide a minimally invasive treatment option for…
Read MoreSamsara Vision Announces First U.S. Surgeries of the SING IMT™ (Smaller-Incision New-Generation Implantable Miniature Telescope), for Age-Related Macular Degeneration as part of the CONCERTO Study
Samsara Vision, a company focused on bringing vision and freedom back to patients with late-stage, age-related macular degeneration (AMD) through advanced visual prosthetic devices, announced the completion of the first U.S. surgeries of its SING IMT™ (Smaller-Incision New-Generation Implantable Miniature Telescope), as part of the CONCERTO clinical study, a U.S.-based Food and Drug Administration (FDA)…
Read MoreWhat’s Your Six Word Story?
If you ever studied English literature, it’s almost certain that you’ve heard of a Six Word Story; A contributing method to Ernest Hemingway’s Iceberg theory in which the writer uses only six words to tell an entire story. You may have even written a few. According to legend, Hemingway constructed the six-word story at lunch…
Read Moreannalise.ai appoints proven global healthcare leader as CEO, officially launches US team following FDA clearances
annalise.ai, the global radiology AI company with rapidly growing presence in Asia- Pacific, Europe and the United Kingdom, today announced the appointment of accomplished healthcare technology executives Lakshmi Gudapakkam as Chief Executive Officer and clinical strategist Dr Rick Abramson as Chief Medical Officer. With the new appointments, annalise.ai expects to further accelerate its global market presence and entry into the US…
Read More