Archive for December 2019
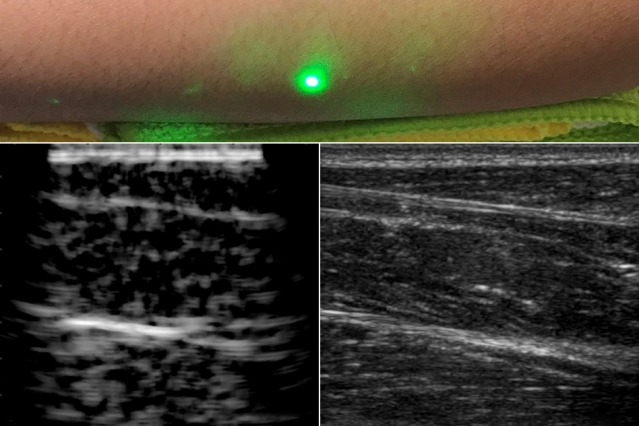
MIT Researchers Produce First Laser Ultrasound Images of Humans
For most people, getting an ultrasound is a relatively easy procedure: As a technician gently presses a probe against a patient’s skin, sound waves generated by the probe travel through the skin, bouncing off muscle, fat, and other soft tissues before reflecting back to the probe, which detects and translates the waves into an image…
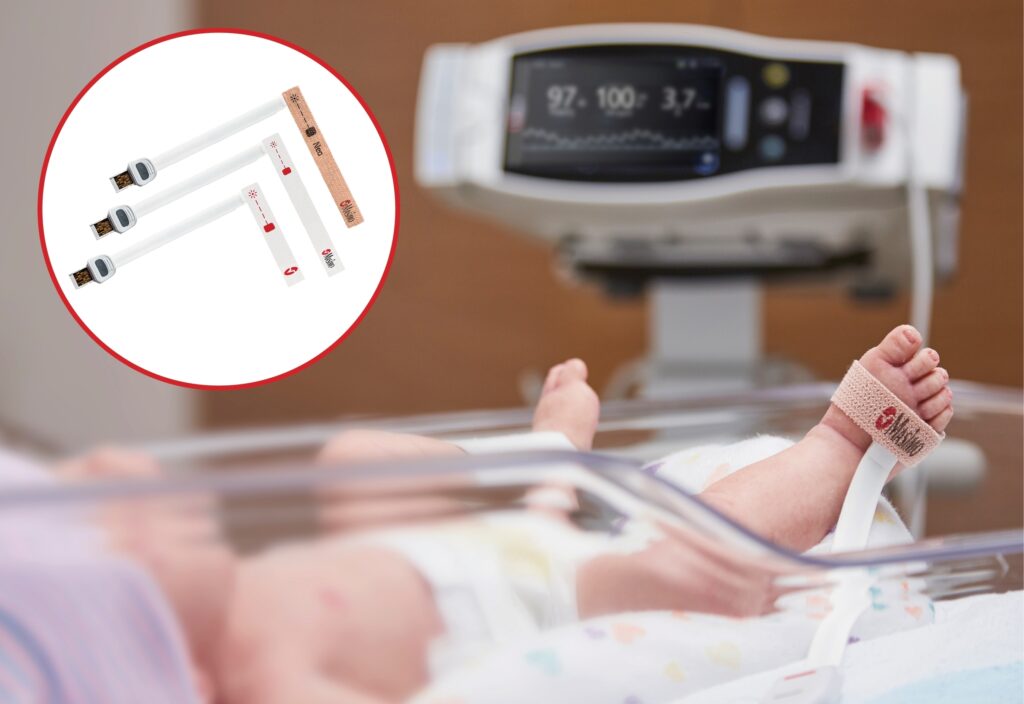
Read MoreMasimo Announces FDA Clearance for Neonatal RD SET Pulse Oximetry Sensors with Improved Accuracy Specifications
Masimo announced today that RD SET® sensors with Masimo Measure-through Motion and Low Perfusion™ SET® pulse oximetry have received FDA clearance for improved oxygen saturation (SpO2) accuracy specifications for neonatal patients (< 3 kg). The updated RD SET® sensors’ SpO2 accuracy specifications have improved significantly, from 3% to 1.5% ARMS1 (at 1 standard deviation), in conditions of motion and no motion,…
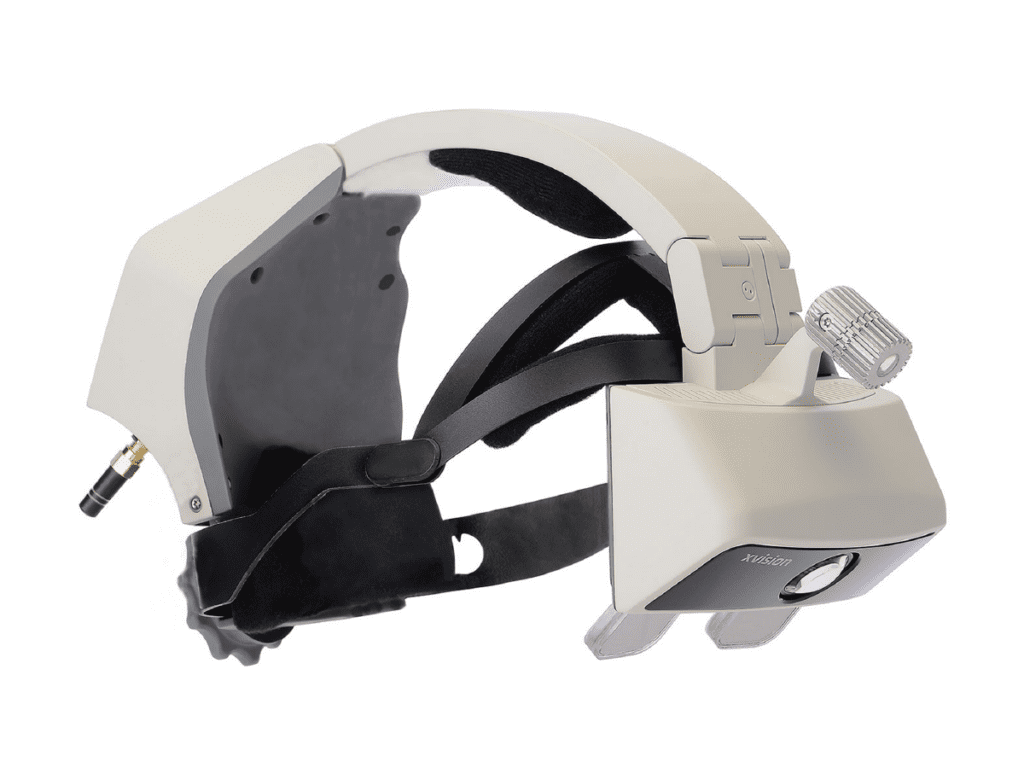
Read MoreAugmedics Announces FDA 510K Clearance and U.S. Launch of xvision, the First Augmented Reality Guidance System for Surgery
Augmedics, a pioneer in augmented reality surgical image guidance, has announced U.S. Food and Drug Administration (FDA) 510(k) clearance and the U.S. launch of its groundbreaking xvision Spine system (XVS), the first AR guidance system to be used in surgery. xvision Spine allows surgeons to visualize the 3D spinal anatomy of a patient during surgery…
Read MoreLife Spine Announces FDA 510(k) Clearance of the Titanium Stand-Alone ALIF Spacer System
Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the Titanium Stand-Alone ALIF Spacer System. “This system clearance is the capstone on a year of incredible product…
Read MoreOpsens Inc. Announces 510(k) Clearance from the FDA to Market its Diastolic Pressure Algorithm
Opsens Inc. announces 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) to market its diastolic pressure algorithm (“dPR”). Coronary physiology has been in constant evolution with the expanded use of Fractional Flow Reserve (“FFR”) and the support of strong clinical data and cardiology societies recommendations. More recently, the option for coronary physiology without hyperemia…
Read MoreIRRAS Receives Renewed CE Mark for the IRRAflow Catheter
IRRAS, a global healthcare company with a comprehensive portfolio of innovative products for neurocritical care, announced today that it received CE Mark approval for its IRRAflow®catheter. This CE Mark complements the two CE Marks previously obtained for the IRRAflow system’s tube set with digital pump and control unit, and allows IRRAS to once again commercially market the IRRAflow system in…
Read MoreBaebies Announces CE Mark for FINDER, an Innovative Near-Patient Testing Platform for Glucose-6-Phosphate Dehydrogenase
Baebies is pleased to announce that FINDERTM, a near-patient testing platform, now has CE Mark as an In Vitro Diagnostic device (IVD) and is commercially available in Europe and other countries that recognize CE Mark. The CE-Marked platform includes an instrument and a cartridge, which tests for Glucose-6-Phosphate Dehydrogenase (G6PD) from low blood volume, a single drop…
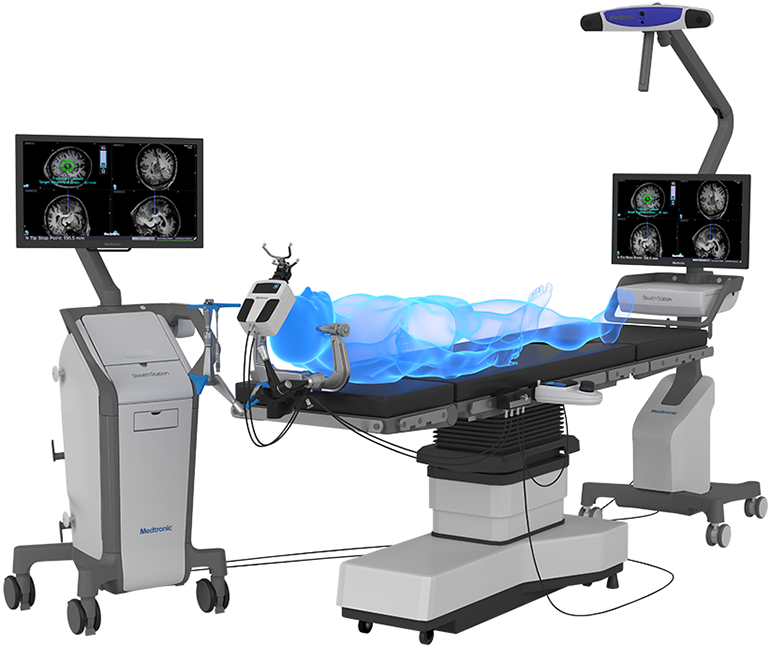
Read MoreMedtronic Expands Surgical Synergy(SM) with FDA Clearance of the Stealth Autoguide System for Cranial Procedures
Medtronic plc announced that the U.S. Food and Drug Administration (FDA) recently cleared the Stealth Autoguide™ system, the first cranial robotic platform that integrates with Medtronic’s enabling technology portfolio to create an end-to-end procedural solution. The Stealth Autoguide Platform is a robotic guidance system intended for the spatial positioning and orientation of instrument holders or…
Read MoreClinical Study Utilizes the myTAIHEART Test to Monitor Cell-Free DNA Levels, 2-10 Days Post-Transplantation, in Pediatric Heart Transplant Recipients
TAI Diagnostics, Inc., focused on developing innovative diagnostic tests for monitoring the health of transplanted organs, today announced the publication of “Early Changes in Cell-Free DNA Levels in Newly Transplanted Heart Transplant Patients” in the December 11th open access version of Pediatric Transplantation, the official journal of the International Pediatric Transplant Society. The publication provides results from a prospective…
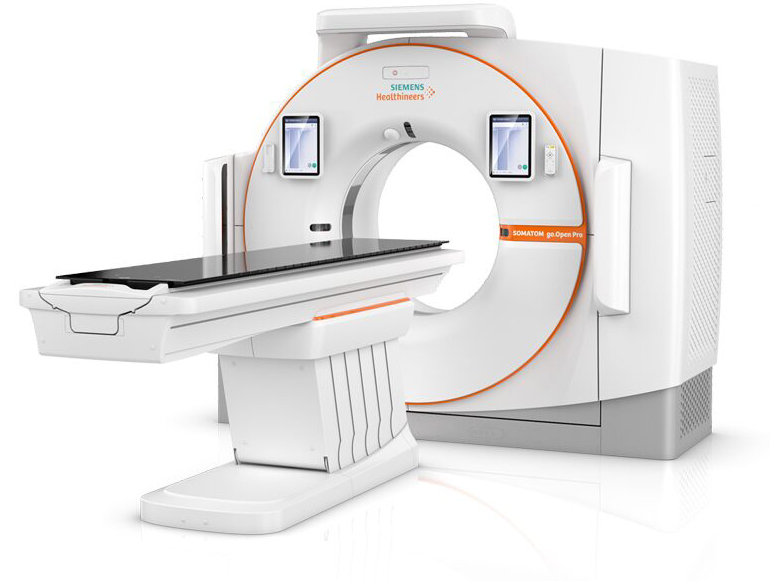
Read MoreFDA Clears Two Siemens Healthineers CT Systems Dedicated for Radiation Therapy Planning
The U.S. Food and Drug Administration (FDA) has cleared the SOMATOM go.Sim and SOMATOM go.Open Pro – two computed tomography (CT) systems from Siemens Healthineers that are dedicated for radiation therapy (RT) planning. The 64-slice SOMATOM go.Sim and 128-slice SOMATOM go.Open Pro are members of the SOMATOM go. platform, which was created to limit potential…
Read More