Archive for November 2019
EOS Imaging Announces the Upcoming Launch of EOSedge, Its New Generation Imaging System
EOS imaging, a leader in 2D/3D orthopedic medical imaging and software solutions for 3D anatomical modeling and surgical planning, announced the launch of its new generation imaging system, EOSedge, in Europe, Canada and Australia. The Company will unveil its new system at the upcoming Radiological Society of North America (RSNA) 2019 Annual Meeting starting on…
Read MoreUS Medical Innovations Receives FDA 510(k) Clearance for the Canady Plasma Smart XL-1000 Generator
US Medical Innovations, LLC (USMI), a Biomedical and Life Science subsidiary of US Patent Innovations, LLC (USPI) announced today the U.S. Food And Drug Administration (FDA) 510(k) clearance (#K192124) to market and sell the Canady Plasma® Smart XL-1000™ Electrosurgical Generator (CPSXEG) with accessories. The CPSXEG combines with a high frequency voltage to electrically enhanced plasma…
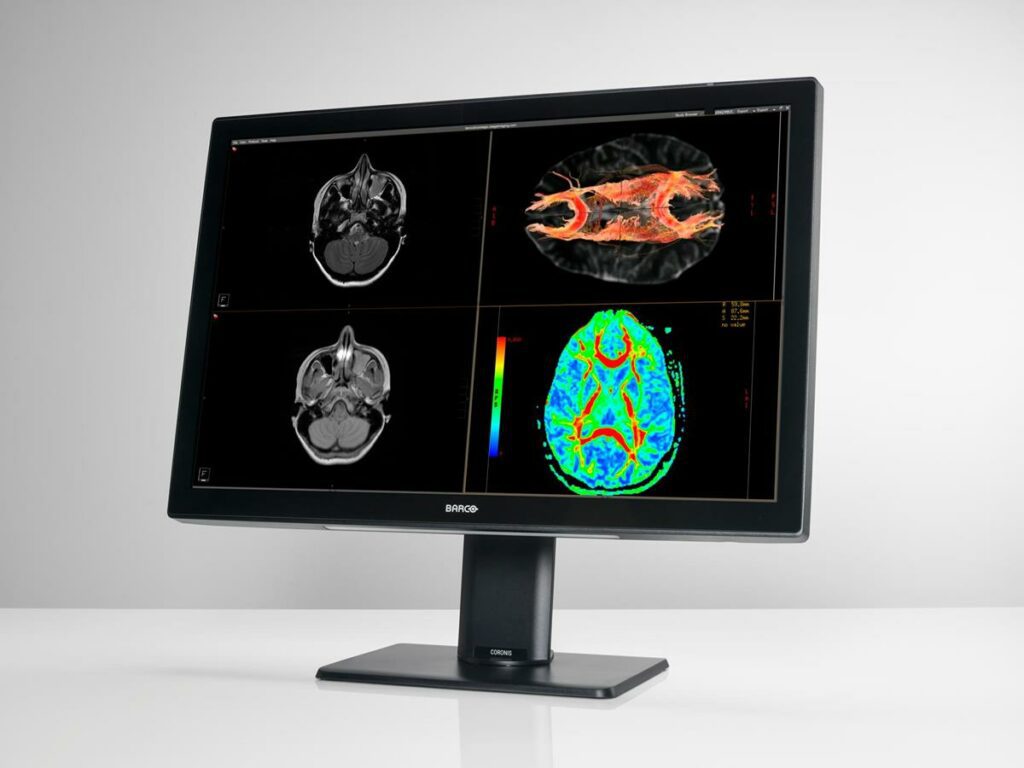
Read MoreBarco to Reveal the Newest Coronis Fusion Display Systems, Perfected for Color Imaging
Barco’s renowned multimodality display for radiologists, Coronis Fusion, now comes with calibrated and consistent color, improved energy efficiency, and lightweight design, along with clinical tools to increase accuracy and decrease reading times. This year at RSNA, booth 1329, Barco will showcase the new Coronis Fusion 6MP and 4MP solutions. What makes the new Coronis Fusion…
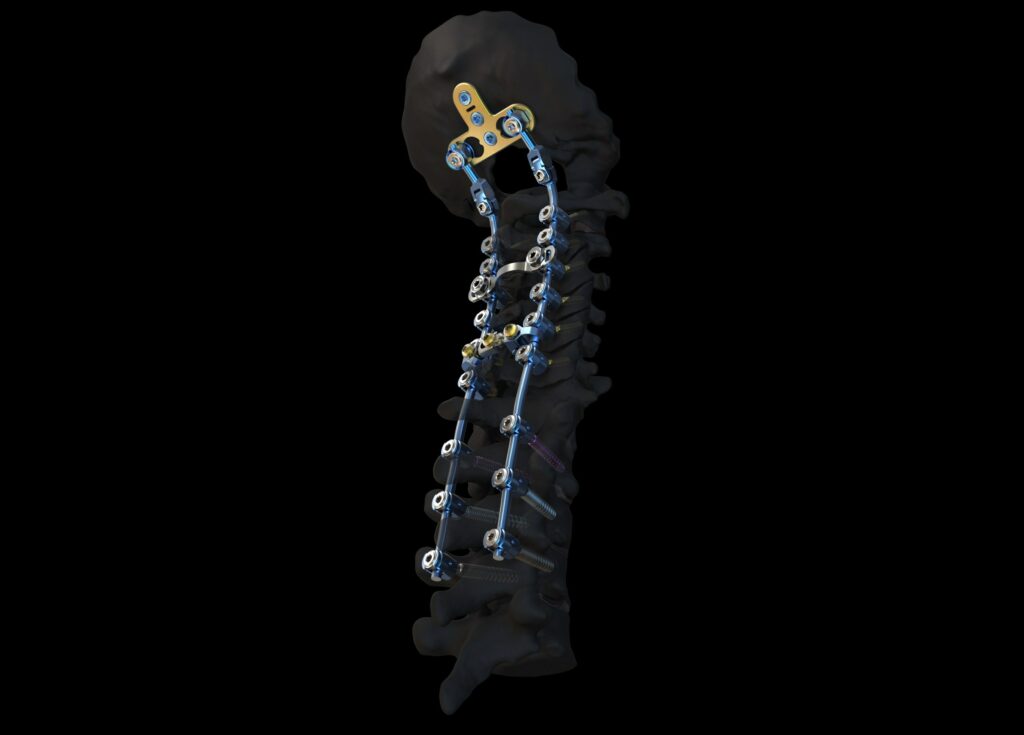
Read MoreNew Cervical Spine System from DePuy Synthes Advances Treatment Options for Patients with Complex Cervical Spine Disorders
The Johnson & Johnson Medical Devices Companies* today announced that DePuy Synthes has launched the SYMPHONY Occipito-Cervico-Thoracic (OCT) System, expanding its offering for the surgical treatment of conditions in the neck and upper back. The SYMPHONY System includes a differentiated offering of instruments and implants designed for stabilization of the spine in patients undergoing Posterior…
Read MorePENTAX Medical Launches DEC HD Duodenoscope in the United States
PENTAX Medical, a healthcare industry leader in diagnostic and therapeutic endoscopy, has announced the United States launch of its DEC™ HD Duodenoscope, an advanced, high-definition duodenoscope that features multiple disposable components, including the sterile distal cap and elevator lever, for unit reprocessing. The DEC HD Duodenoscope is designed to provide physicians a solution aligned with recent…
Read MoreHologic to sell Cynosure medical aesthetics business for $205m
Hologic, Inc. announced that it has entered into a definitive agreement to sell its Cynosure medical aesthetics business to an affiliate of investment funds managed by Clayton, Dubilier & Rice for a total purchase price of $205 million in cash, subject to certain closing adjustments. Net of these adjustments, Hologic expects net cash proceeds of…
Read MoreSynaptic Medical Appoints Robert Ricker as President of US Commercialization
LAKE FOREST, Calif., October 24, 2019 – Synaptic Medical, a medical device company that focuses on developing breakthrough products for the treatment of arrhythmias and other cardiovascular diseases, today announced that Robert C. Ricker has been appointed President of US Commercialization. Mr. Ricker’s broad executive experience in the cardiovascular industry are invaluable to the future…
Read MoreData Show Zilver PTX Leads to Fewer Complications and Shorter Hospital Stays Than Traditional Bypass Surgery
At this year’s VEITHsymposium, Dr. Marc Bosiers presented data that show that patient treatment with the Zilver PTX stent has several benefits when compared to traditional bypass surgery. The data, which were gathered from a randomized controlled trial, show that treatment with Zilver PTX results in fewer complications and shorter hospital stays for patients with…
Read MoreNanox Introduces New Digital X-ray Technology That Could Significantly Reduce the Price of Medical Imaging Systems
Nano-X Imaging Ltd. a medical imaging technology company, introduces the first commercial-grade digital X-ray technology based on a proprietary silicon MEMs semiconductor technology. This technology is introduced after 15 years of “under the radar” Japanese and Israeli development and substantial investment to overcome one of the biggest barriers to modern medical imaging. This novel digital X-ray source may enable…
Read MoreBioVentrix Gains FDA Breakthrough Device Designation for their Revivent TC Transcatheter Ventricular Enhancement System
BioVentrix, Inc., a privately-held company with a first-in-class, transcatheter-based structural heart device to treat heart failure, today announced that the U.S. Food and Drug Administration (FDA) has granted the company Breakthrough Device Designation status for its Revivent TC™ Transcatheter Ventricular Enhancement System for heart failure. Less Invasive Ventricular Enhancement, or the LIVE™ procedure, uses the…
Read More