Archive for December 2022
Endoluxe Announces First ENT Procedures Using Endoluxe Vision System
Endoluxe congratulates Keith Matheny, MD, FARS, of Collin County Ear Nose & Throat in Frisco, TX, for being the first ear, nose and throat (ENT) surgeon in the U.S. to use the Endoluxe Vision System for endoscopic sinus surgery. Dr. Matheny is a nationally-known innovator in the field of Otolaryngology and has developed or been…
Read MoreInspira™ Technologies Begins Manufacturing of the ALICE™ CPB Device, ahead of planned 2023 FDA submission
Inspira Technologies OXY B.H.N. Ltd., a groundbreaking respiratory support technology company, announced today that it has begun the manufacturing process for the ALICE CPB (Cardiopulmonary Bypass) device (the “ALICE device”) to undergo the Verification and Validation phase prior to its planned 2023 submission to the U.S. Food and Drug Administration (FDA) for 510(k) clearance. If FDA…
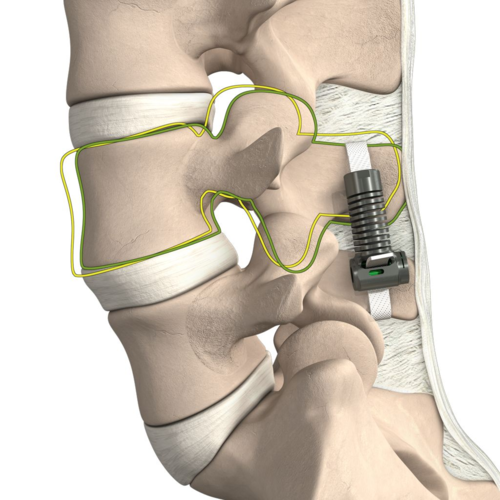
Read MoreEmpirical Spine Completes Full Premarket Approval (PMA) Submission to FDA for Limiflex™ Dynamic Sagittal Tether™
Empirical Spine, Inc., a medical device company creating a new class of spinal implant, recently completed the final step in the U.S. Food & Drug Administration (FDA) submission process for the LimiFlex™ Dynamic Sagittal Tether™ (DST). The Premarket Approval (PMA) submission included Module III, with data and analysis of the two-year results from the pivotal Investigational Device…
Read MoreSonorous NV Uses New Device to Treat Patients with Symptomatic Cerebral Venous Diseases
Sonorous NV, Inc. Chairman, Dave Ferrera, announced treatment of the first patients in North America with the BossStent® device designed to treat patients with symptomatic cerebral venous diseases. The single-use, implantable device acts as an endoluminal prothesis that is placed into a cerebral venous sinus stenosis to normalize blood flow and pressure gradients causing pulsatile tinnitus and/or IIH (idiopathic intracranial hypertension),…
Read MoreMovano Health On Track to File First FDA Submission for its Smart Ring’s SpO2 and Heart Rate Data Following Successful Pivotal Hypoxia Trial
Movano Health, a purpose-driven healthcare solutions company at the intersection of medical and consumer devices, today announces successful preliminary results of its pivotal hypoxia trial, which was completed in conjunction with the University of California, San Francisco (UCSF) to assess the accuracy of its smart ring’s blood oxygen saturation (SpO2) and heart rate data. In comparing the overall accuracy…
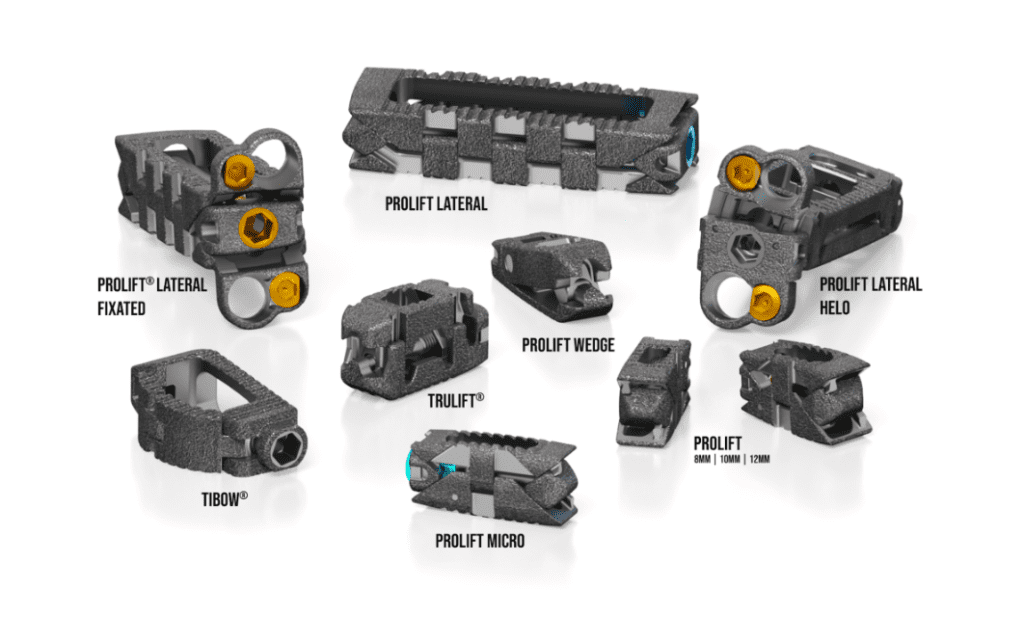
Read MoreLife Spine Announces FDA 510(K) Clearance for the TruLift® Lateral Expandable Spacer System and Lateral Plate System
Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the TruLift Lateral Expandable Spacer System and Lateral Plate System. Utilizing expandable technology and robust plating options, TruLift Lateral…
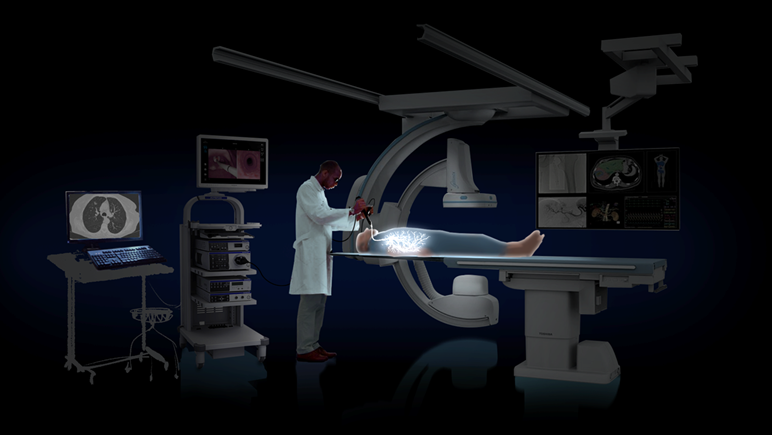
Read More2,000 AI-Driven, Intraoperative CT-Guided Procedures Completed with LungVision
Body Vision Medical, a leader in AI-driven, intraoperative imaging, announced that its LungVision™ AI-driven, intraoperative CT imaging system has now been used in 2,000 diagnostic bronchoscopy procedures worldwide. Body Vision Medical’s LungVision™ System uses artificial intelligence (AI) to transform X-ray images from any C-arm into real-time, intraoperative CT scans. This empowers bronchoscopists to biopsy from smaller, more…
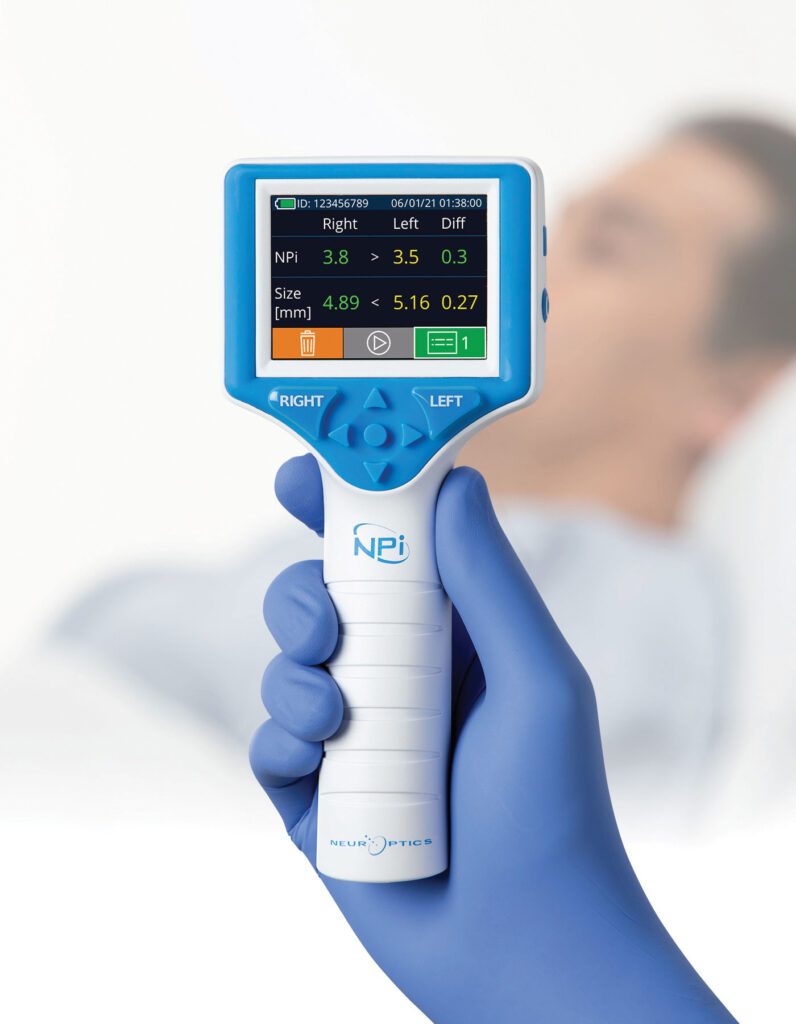
Read MoreNeurOptics’ Neurological Pupil index™ and Automated Pupillometry Included in Latest European Guidelines for Post-Resuscitation Care
NeurOptics’ Neurological Pupil index™ (NPi®) and automated pupillometry are now included in the updated 2021 European Resuscitation Council (ERC) and European Society of Intensive Care Medicine (ESICM) Guidelines for post-resuscitation care as an objective measurement supporting neurological prognosis in patients following cardiac arrest. The ERC and ESICM guidelines outline the latest European post-resuscitation care science…
Read MoreIRRAS Receives US FDA 510(k) Clearance for its Next Generation IRRAflow Control Unit
IRRAS, a commercial-stage medical technology company with a comprehensive portfolio of innovative products for neurocritical care, announced that it received regulatory clearance from the United States Food, Drug, and Administration (FDA) to active all of the functionality on the next generation control unit for its flagship product, IRRAflow, the world’s first and only active fluid…
Read MorePennsylvania-based Highmark Inc. Joins Theranica’s Coverage with Evidence Program for Nerivio® Drug-Free Migraine Treatment Device
Theranica, a prescribed digital therapeutics company developing advanced neuromodulation devices for migraine and other pain conditions, announced that Highmark Inc. (Highmark), one of America’s leading health insurance organizations and an independent licensee of the Blue Cross Blue Shield Association, will conduct a targeted Coverage with Evidence program with the goal of providing its members with affordable…
Read More