Archive for September 2023
The FDA Approves IDE for ReGelTec’s Pivotal Study of HYDRAFIL® for Chronic Low Back Pain due to Degenerative Disc Disease
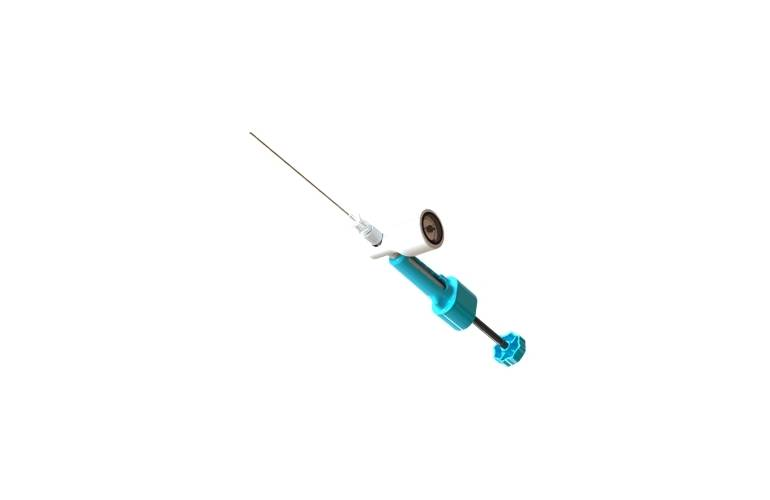
ReGelTec, Inc., announced that the U.S. Food and Drug Administration has approved an IDE for the company’s pivotal study to support premarket approval of its HYDRAFIL® System. The HYDRAFIL System contains an injectable polymer that is implanted percutaneously via a needle to augment the native disc in a procedure performed under local anesthesia at an…
Read More4WEB Medical Receives 510k Clearance to Market New Integrated Cervical Plate
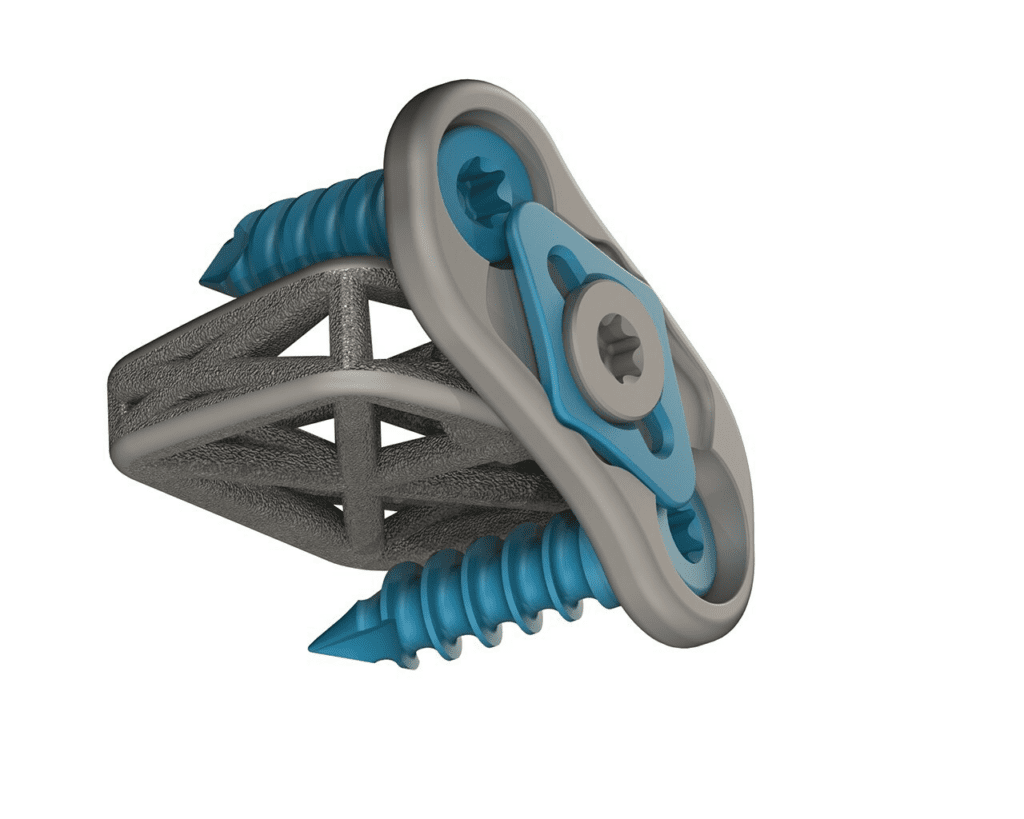
4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, announced that it has received 510K clearance to market the newest additions to the company’s implant portfolio, the Cervical Spine Truss System (CSTS) Integrated Plating Solution and two cervical interbody line extensions which include implants with 12 degrees of…
Read MoreQT Medical® Announces Completion of Series B Financing of $12 Million
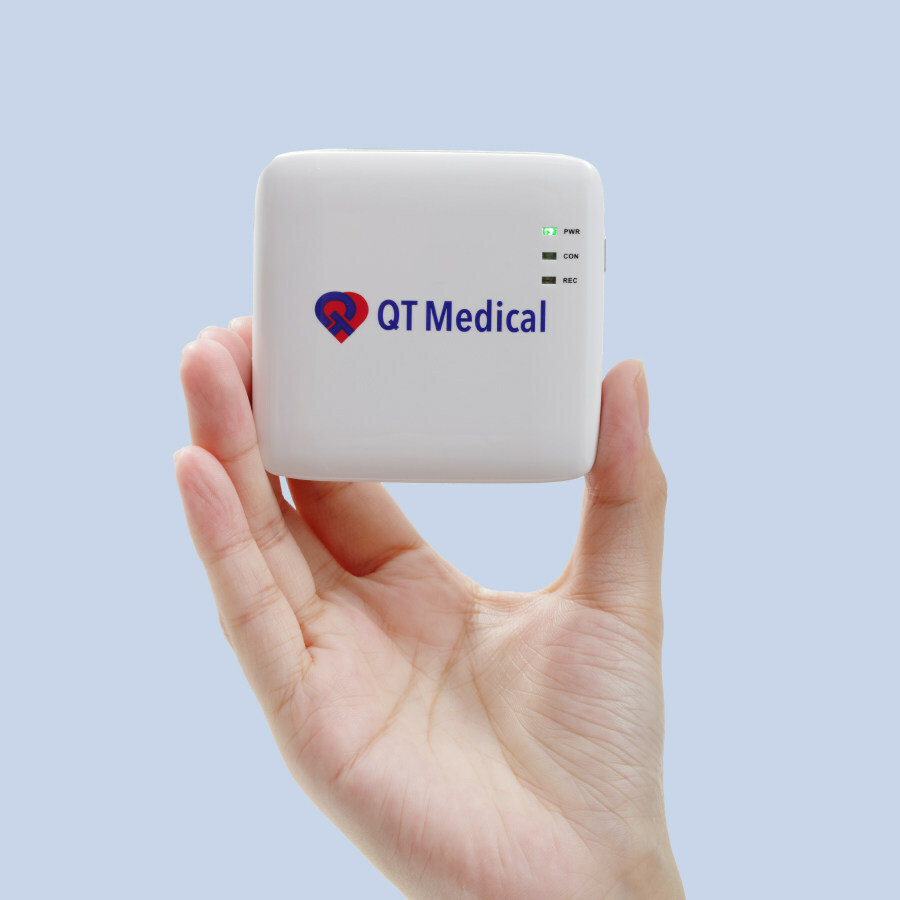
QT Medical, Inc., a medtech company focused on cardiac care technology, announced today that it raised $12 million in Series B financing. This funding will accelerate QT Medical’s market penetration, global expansion, product pipeline development, and long-term strategic planning. QT Medical aims to provide improved cardiac devices and services to revolutionize the care of patients with heart disease.…
Read MoreSaluda Medical Receives FDA Approval for Evoke® System MRI Labeling
Saluda Medical, Inc. (“Saluda Medical”), a global medical device company revolutionizing the field of neuromodulation with an emerging portfolio of therapies driven by advanced closed-loop technologies, today announced that the U.S. Food and Drug Administration (FDA) has approved MRI conditional labeling for the Evoke® System, the first and only precision, dose-control spinal cord stimulation (SCS) therapy…
Read MoreChoiceSpine® Announces Standalone Indication for Blackhawk® Ti 3D Printed Cervical Spacer System
ChoiceSpine LLC, a privately held spinal device company held by Altus Capital Partners in Knoxville, TN, is pleased to announce today that it has received clearance from the U.S. Food and Drug Administration (FDA) to market the Blackhawk® Ti 3D Printed Cervical Spacer System with standalone clearance. Blackhawk® Ti utilizes preassembled integrated anchor technology with a proven…
Read MoreSpectraWAVE Secures 510(k) Clearance to Add Saline Imaging and Expanded Artificial Intelligence Features to the HyperVue™ Imaging System
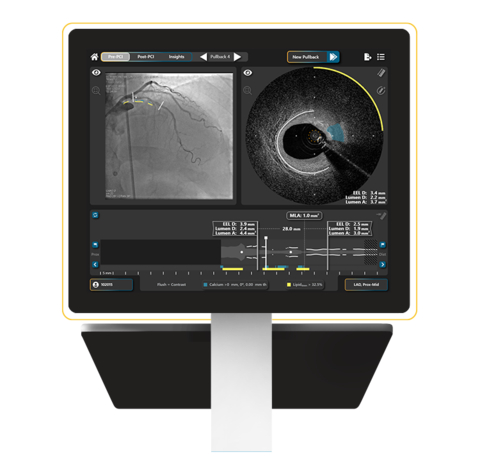
SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for patients with coronary artery disease (CAD), today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for product enhancements to the HyperVue™ Imaging System. The intravascular imaging system combines next-generation DeepOCT™ images and near infrared spectroscopy (NIRS) with state-of-the-art ease of…
Read MoreCereVasc Receives FDA IDE Approval to Expand its Clinical Study of Patients with Normal Pressure Hydrocephalus Utilizing the Generation 2 eShunt® System
CereVasc, Inc., a clinical-stage, medical device company developing novel treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) supplement to permit the expansion of the study of the eShunt System in patients with Normal Pressure Hydrocephalus (NPH) to additional study participants and clinical sites.…
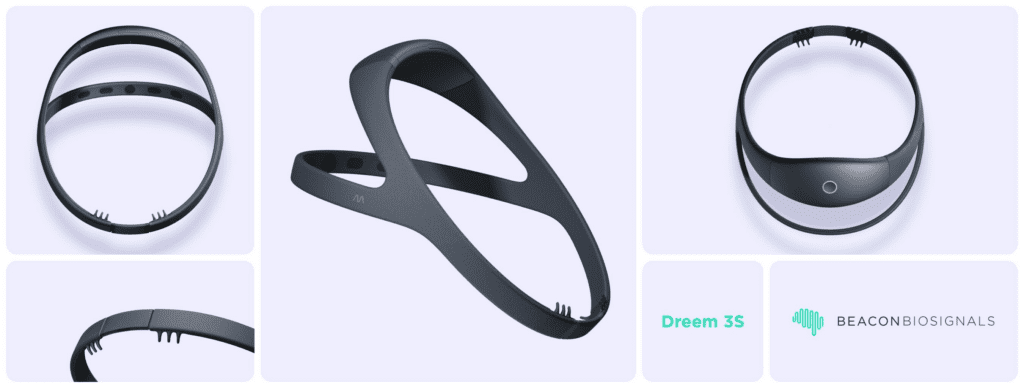
Read MoreBeacon Biosignals Receives FDA Clearance for AI-Assisted Sleep Monitoring Device Dreem 3S
Beacon Biosignals is thrilled to announce the milestone achievement of FDA 510(k) Clearance for the Dreem 3S, an advanced wearable headband with integrated machine learning algorithms to capture electroencephalogram (EEG) data from the brain to monitor sleep architecture and aid in the diagnosis of disturbed sleep. FDA Clearance marks it as equivalent to in-lab polysomnography for the…
Read MoreInspira™ Announces 510(k) FDA Submission of INSPIRA™ ART100 Towards Commercialization
Inspira™ Technologies OXY BHN Ltd. (Nasdaq: IINN, IINNW) (the “Company” or “Inspira Technologies”), a company aiming to bring a paradigm shift to acute respiratory care by empowering breathing without lungs, announced it had submitted its INSPIRA ART 100, a cardio-pulmonary bypass device, to the U.S. Food and Drug Administration (FDA) via the 510(k) pathway, with potential clearance…
Read MoreOrthofix Announces Leadership Changes
Orthofix Medical Inc. (NASDAQ: OFIX), a leading global spine and orthopedics company, today announced that Catherine Burzik, Chair of the Orthofix Board of Directors, has been appointed Interim Chief Executive Officer; Geoffrey Gillespie, Orthofix Vice President, Corporate Controller, has been appointed Interim Chief Financial Officer; and Puja Leekha, Orthofix Senior Vice President, Chief Ethics and…
Read More