Archive for September 2023
FDA Approves LimFlow System in Patients With Chronic Limb-Threatening Ischemia and No Suitable Endovascular or Surgical Revascularization Options
LimFlow SA, a pioneer in the development of minimally-invasive technology for the treatment of chronic limb-threatening ischemia (CLTI), a severe form of peripheral artery disease (PAD), announced today that the U.S. Food and Drug Administration (FDA) has approved the LimFlow System to help people with CLTI who have no other suitable endovascular or surgical treatment…
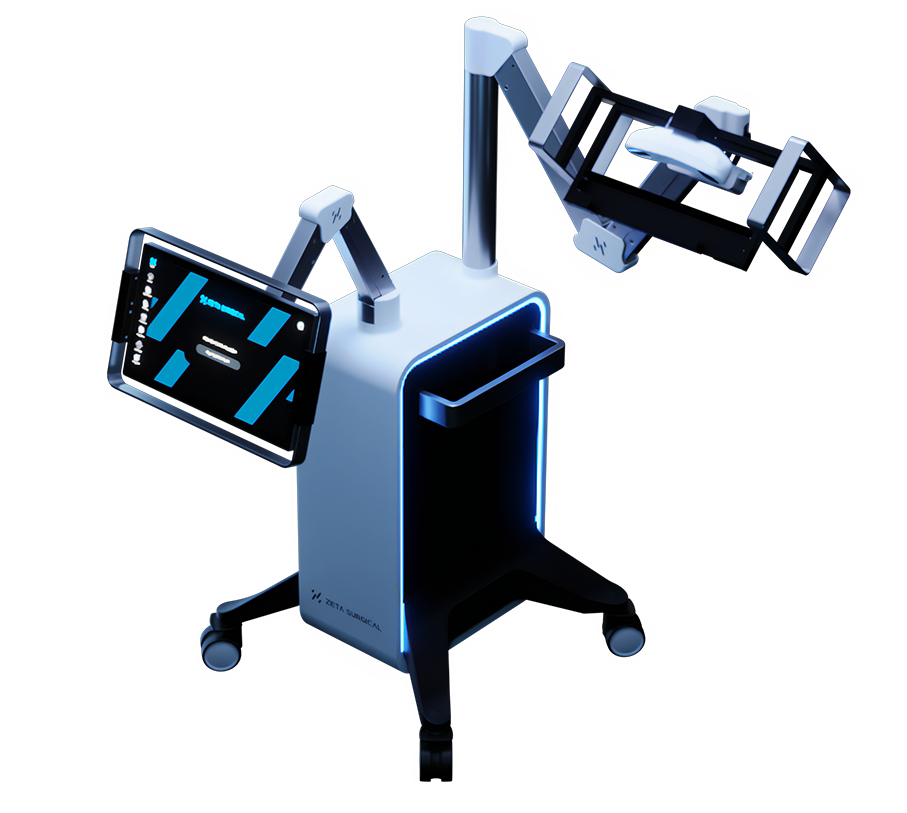
Read MoreZeta Surgical’s Mixed Reality Navigation System Receives FDA Clearance
Zeta Surgical, a surgical robotics and mixed reality company, announced today that the U.S. Food and Drug Administration has cleared the Zeta Cranial Navigation System, its mixed reality surgical navigation system. The Zeta Cranial Navigation System is a mixed-reality navigation system for neurosurgery that provides surgeons with “GPS-like” guidance with millimetric accuracy in real time. Zeta’s…
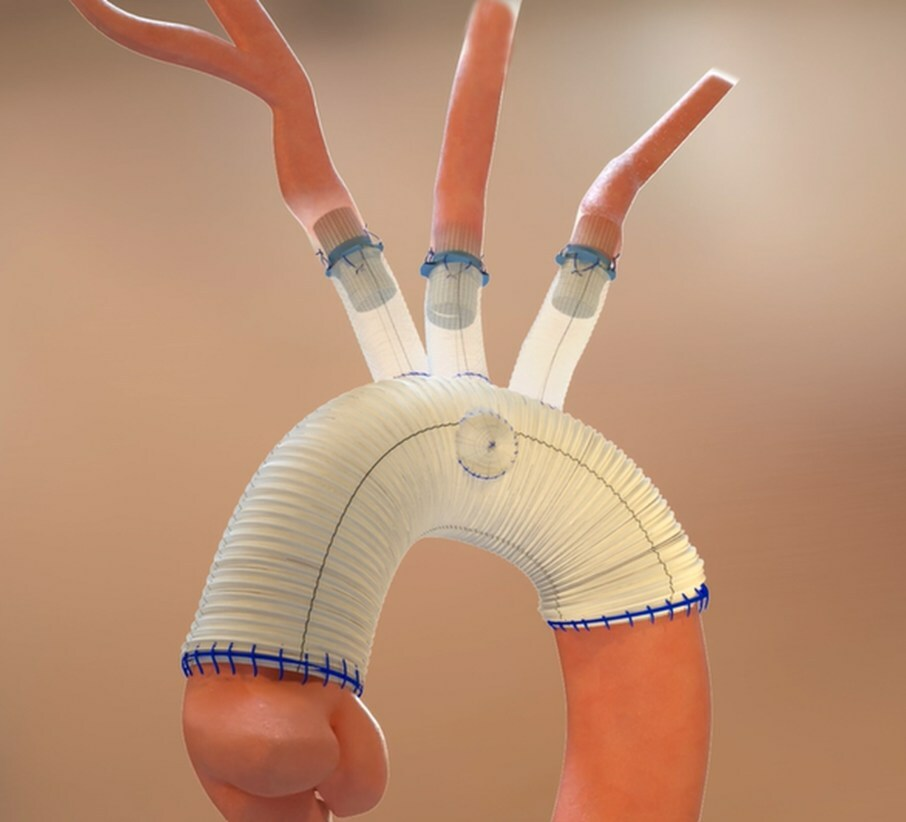
Read MoreAquedeon Medical, Inc. Receives FDA IDE Approval for the Duett Vascular Graft System
Aquedeon Medical, Inc., a Silicon Valley pioneering medical device company specializing in novel cardiothoracic solutions, is pleased to announce a significant milestone following receipt of FDA Investigational Device Exemption (IDE) approval to conduct a staged pivotal clinical trial for its Duett Vascular Graft System in the United States. The study will be initiated in the second…
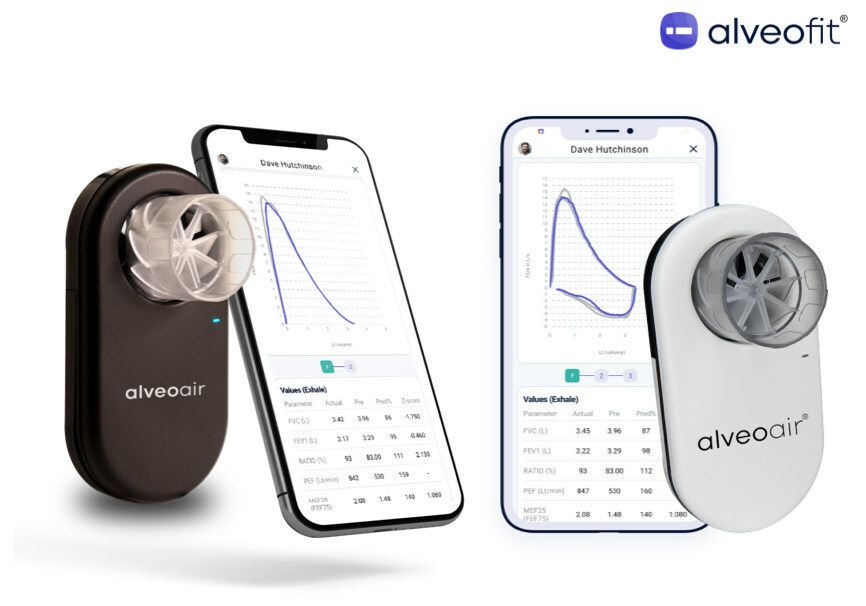
Read Morealveoair® has received U.S. FDA clearance, marking a significant advancement in respiratory care
alveofit® (Roundworks Technologies Private Limited) is thrilled to announce that its groundbreaking product, alveoair® spirometer, has earned U.S. FDA clearance for distribution in the United States. Specializing in digital therapeutics for respiratory care, the company is committed to delivering affordable and interoperable lung health solutions. This U.S. FDA approval, coupled with strategic partnerships like the one…
Read MoreAbbott to Acquire Bigfoot Biomedical, Furthering Efforts to Develop Personalized, Connected Solutions for People with Diabetes
Abbott (NYSE: ABT) and Bigfoot Biomedical today announced a definitive agreement for Abbott to acquire Bigfoot, a leader in developing smart insulin management systems for people with diabetes. The transaction is subject to customary closing conditions and is expected to close in the third quarter of 2023. Financial terms were not disclosed. Abbott and Bigfoot have worked together on connected diabetes solutions since…
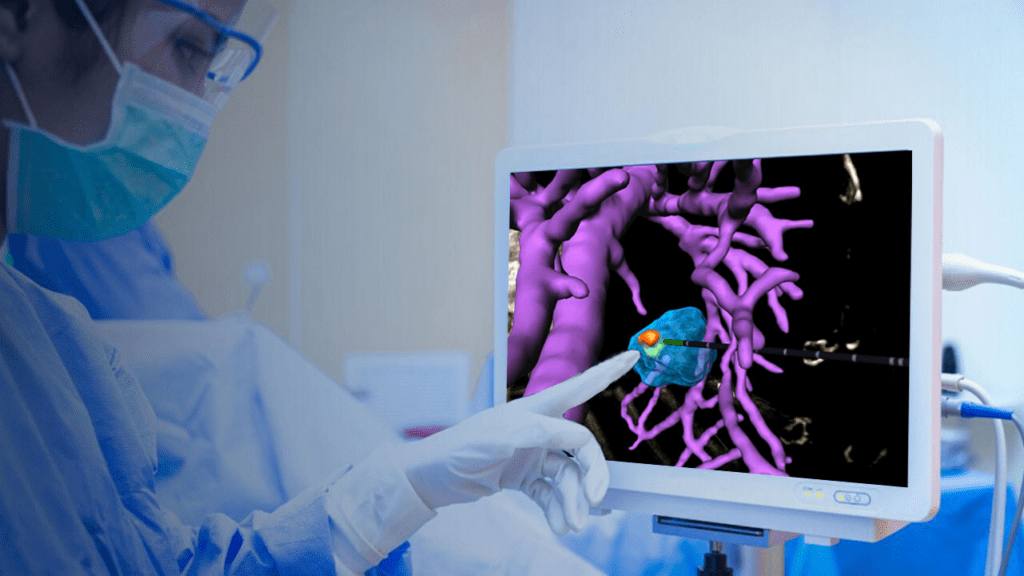
Read MoreTechsomed Announces FDA Clearance for Ablation Treatment Planning and Confirmation Software
Techsomed Ltd., a medical software innovator dedicated to enhancing clinical impact in ablation therapy, announced today that it has received 510(k) clearance from the USA Food and Drug Administration (FDA) for its VisAble.IO software intended to assist physicians in planning liver ablation procedures, and confirming ablation zones, with the goal of increasing treatment precision. Ablation…
Read More