Archive for September 2022
Pulse Biosciences Announces FDA 510(k) Clearance for the Treatment of Sebaceous Hyperplasia
Pulse Biosciences, Inc. (Nasdaq: PLSE), a novel bioelectric medicine company developing the CellFX® System powered by Nano-Pulse Stimulation™ (NPS™) technology, announced receipt of U.S. Food and Drug Administration (FDA) 510(k) clearance for its CellFX System, expanding the indication for use to include the treatment of sebaceous hyperplasia in patients with Fitzpatrick skin types I-III. This…
Read MoreFDA Grants QT Imaging™ Clearance to Calculate Fibroglandular Volume of the Breast
The U.S. Food and Drug Administration (FDA) has granted QT Imaging, Inc. 510(K) clearance to calculate the fibroglandular volume (FGV) of the breast and the ratio of FGV to total breast volume (TGV). This ratio can contribute to an assessment of risk for breast cancer and changes in this ratio can be used to measure…
Read MoreOpSens Announces FDA Clearance for the SavvyWire™ for Use in Transcatheter Aortic Valve Replacement (TAVR) Procedures
OpSens Inc., a medical device cardiology-focused company delivering innovative solutions based on its proprietary optical technology, announced that it has received 510(k) regulatory clearance from the U.S. Food & Drug Administration (“FDA”) for the SavvyWire™ (“SavvyWire”), its new guidewire for transcatheter aortic valve replacement procedures, or TAVR. “For OpSens, FDA clearance is a key milestone…
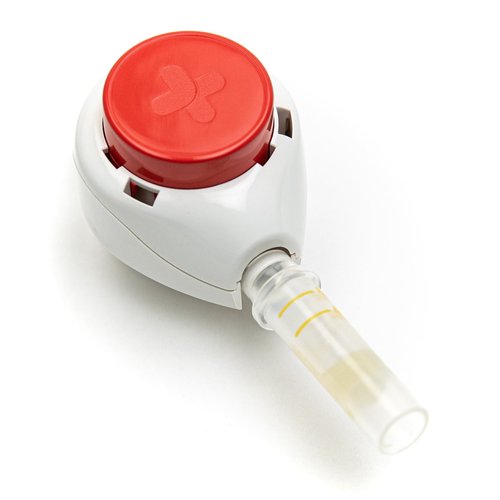
Read MoreTasso+™ Device Earns FDA 510(k) Class II Medical Device Clearance
Tasso, Inc., the leading provider of convenient, clinical-grade blood collection solutions, announced that the U.S. Food and Drug Administration (FDA) has cleared its Tasso+™ lancet as a Class II medical device. The clearance allows the company to market and sell the device to more pharmaceutical companies, healthcare organizations, and academic institutions across the country, expanding…
Read MorePotrero Medical Receives FDA Breakthrough Device Designation for Accuryn AKI Predict Algorithm
Potrero Medical announced that the FDA granted Breakthrough Device Designation for their AKI Predict machine learning algorithm, for the advanced prediction of acute kidney injury (AKI) associated with intra-abdominal hypertension (IAH) in cardiac post-surgical intensive care patients. Joe Urban, Potrero CEO stated “We celebrate the breakthrough designation, and we are excited about what this will…
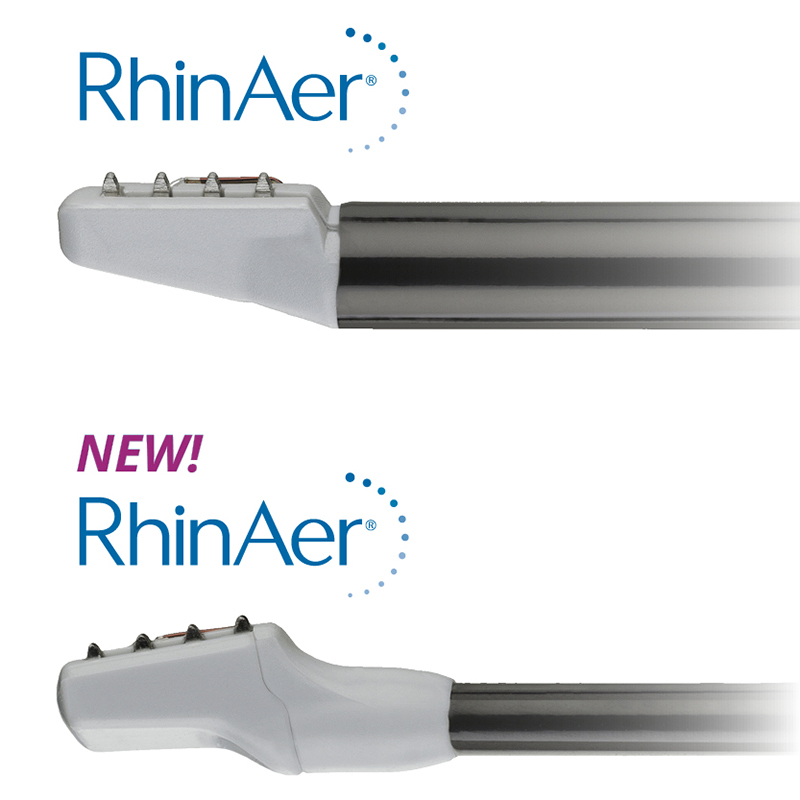
Read MoreAerin Medical Receives FDA Clearance of Next-Generation RhinAer® Stylus for Treatment of Patients with Chronic Rhinitis
Aerin Medical Inc., a company that provides Ear, Nose and Throat (ENT) physicians with non-invasive solutions to treat chronic nasal conditions, announced U.S. Food and Drug Administration (FDA) 510(k) clearance and launch of a next-generation RhinAer® stylus. RhinAer is a non-invasive, temperature-controlled radiofrequency technology that durably treats the causes of rhinorrhea (runny nose), post-nasal drip…
Read MoreSerpex Medical Announces Second FDA 510(k) Clearance of Its Steerable Endobronchial Instrument Platform – The Compass Steerable Needle
Serpex Medical announced U.S. FDA 510(k) clearance of its Compass Steerable Needles – steerable biopsy needles that enable precise access to lung nodules in the intrapulmonary region. Serpex Medical seeks to leverage the power of steerable instruments to enable greater precision and access to improve the diagnosis and treatment of lung cancer. The clearance of…
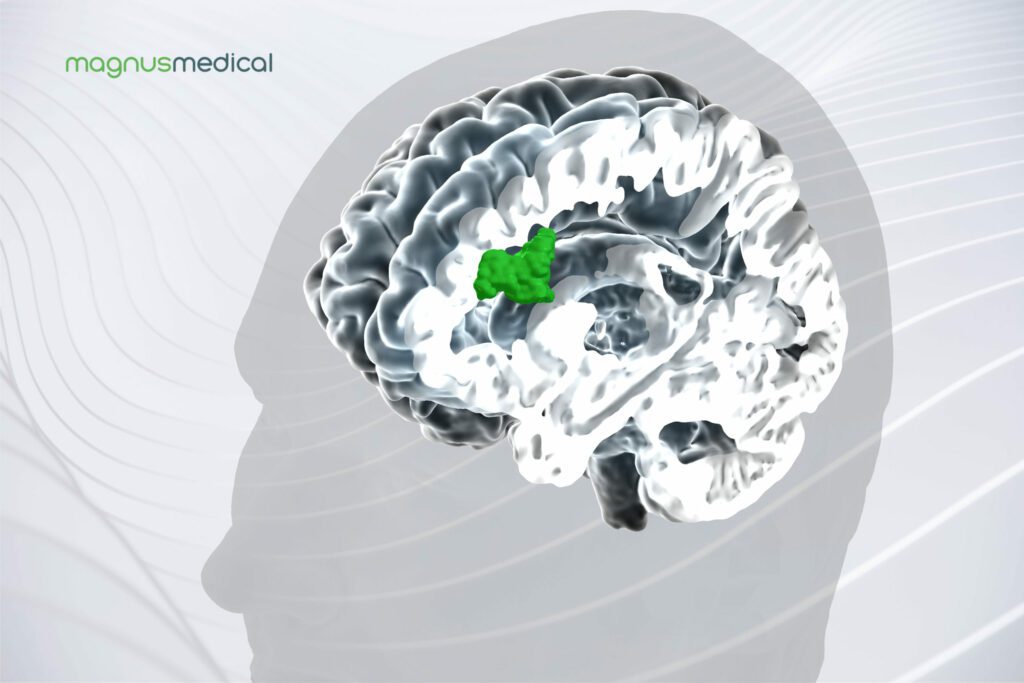
Read MoreMagnus Medical Receives FDA Clearance for the SAINT Neuromodulation System
Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for treatment of neuropsychiatric disorders, announced it received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for the SAINTTM Neuromodulation System for the treatment of major depressive disorder (MDD) in adults who have failed to achieve satisfactory improvement from prior antidepressant…
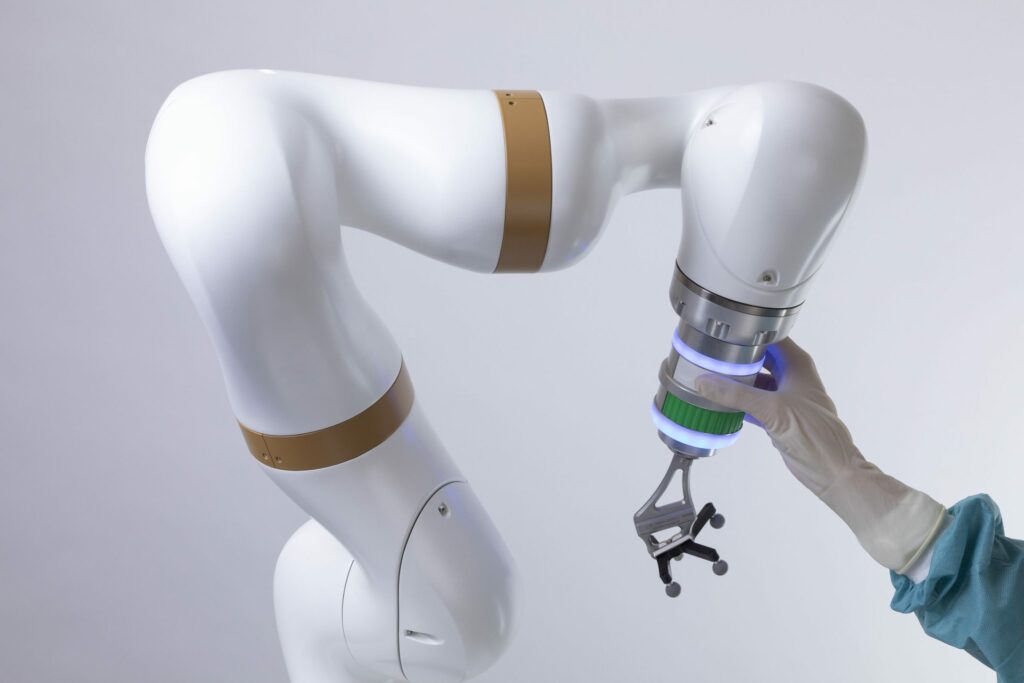
Read MoreeCential Robotics Receives FDA Clearance for its Surgical Robotic Platform for Spine Surgery
eCential Robotics, a French growth MedTech company that designs and produces a system unifying 2D/3D imaging, surgical navigation and robotics, announced FDA 510(k) clearance of its 3D imaging, navigation and robotics guidance system, securing the penetration of its unified robotic platform in the United States. After having recently concluded partnership with US implant companies, eCential Robotics…
Read More