Archive for October 2020
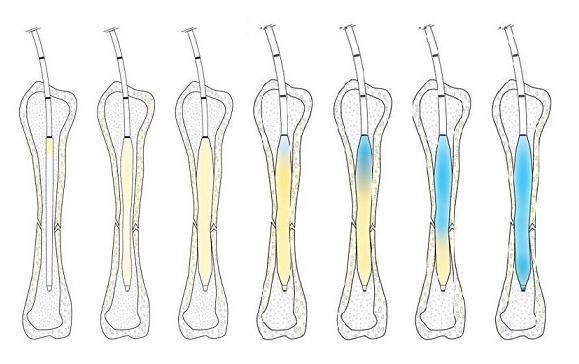
IlluminOss Medical Receives FDA Clearance for Use in Femur and Tibia Fractures as a Supplement to Approved Hardware
IlluminOss Medical, a medical device company focused on minimally invasive orthopedic fracture repair, today announced an expanded U.S. Food and Drug Administration (FDA 510k) clearance for its Photodynamic Bone Stabilization System. The new clearance allows IlluminOss to be used in femur and tibia fractures as supplemental fixation to FDA-cleared fracture fixation systems. “Femur and tibia fractures can…
Read MoreMedical Microinstruments Announces CE Mark for Robotic Microsurgery with the World’s Smallest Wristed Surgical Instruments
MMI SpA, an Italian company dedicated to improving clinical outcomes for patients undergoing microsurgery, announced today the CE Mark, launch and first human use of its Symani® Surgical System in Europe for open microsurgical procedures. The first four robotic surgeries were successfully performed in Florence, Italy, including three complex, post-traumatic lower limb reconstructions as well as a post-oncological reconstruction…
Read MoreSiemens X-Ray System Gains FDA Clearance
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the YSIO X.pree, a ceiling-mounted radiography system with the MyExam Companion intelligent user interface. In addition to this easy-to-use interface that guides the radiologic technologist through the exam workflow, the YSIO X.pree has a new 3D camera for patient positioning and advanced collimation,…
Read MoreBristol Myers Squibb to Acquire MyoKardia for $13.1 Billion in Cash
Bristol Myers Squibb (NYSE: BMY) and MyoKardia, Inc. (Nasdaq: MYOK) today announced a definitive merger agreement under which Bristol Myers Squibb will acquire MyoKardia for $13.1 billion, or $225.00 per share in cash. The transaction was unanimously approved by both the Bristol Myers Squibb and MyoKardia Boards of Directors and is anticipated to close during the…
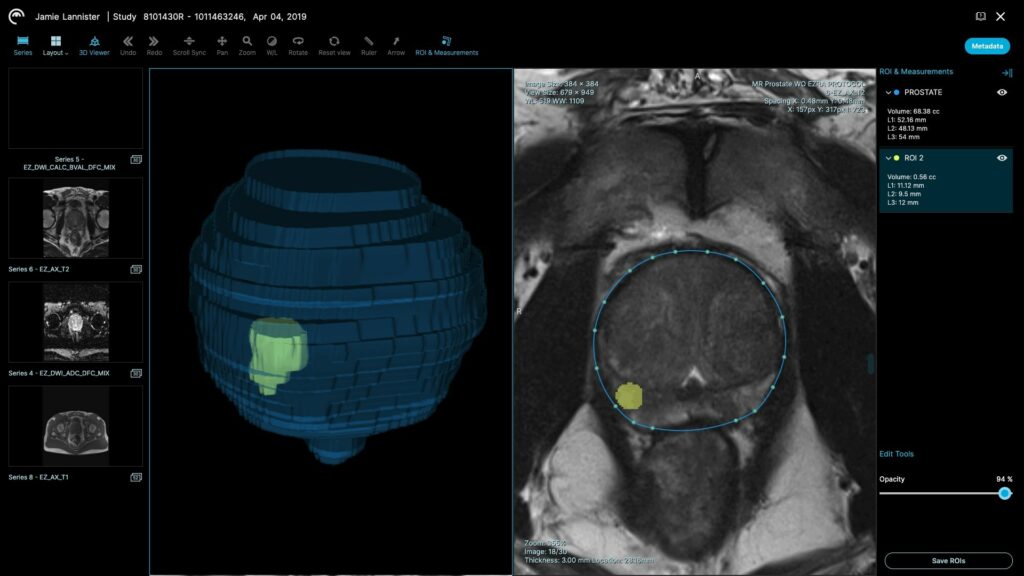
Read MoreEzra Receives FDA Clearance for Prostate Cancer Artificial Intelligence
Ezra, the New York-based startup transforming early cancer screening using MRI, announced today that it has received FDA 510(k) clearance for its Artificial Intelligence designed to assist radiologists in analyzing and segmenting prostate MRI. Use of the innovative AI technology can help reduce the time and cost of MRI-based prostate cancer screening. It is the first…
Read MoreLivmor wins FDA clearance for continuous heart monitoring wearable
LIVMOR, Inc., a leading digital health solutions company, announced today that the Company received FDA 510(k) clearance for the LIVMOR Halo™ AF Detection System, a physician-prescribed wearable solution that provides continuous monitoring of pulse rhythms for the detection of atrial fibrillation (AF), on demand during the day and automatically overnight. Continuous monitoring can significantly enhance the…
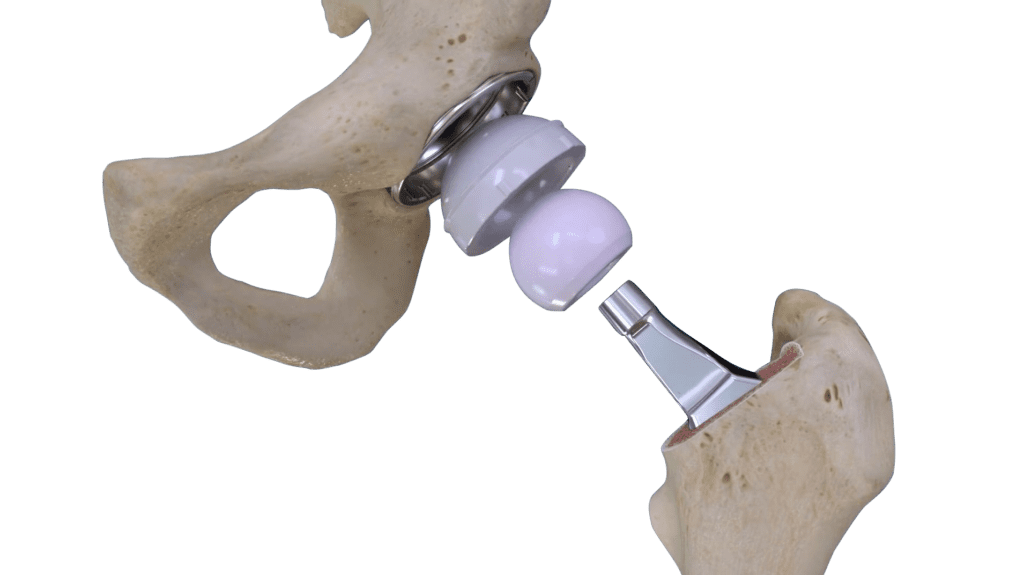
Read MoreConformis Announces 510(k) Clearance for Cordera Hip System
Conformis, Inc. today announced 510(k) clearance by the U.S. Food and Drug Administration of the Company’s new Cordera Hip System. The first product in the Conformis hip product line, the Conformis Hip System, was launched commercially in November 2019 and provided a patient-specific stem. The Cordera Hip System is an uncemented, primary total hip replacement…
Read MoreCrossBay Medical Receives FDA Clearance for CrossGlide ETS Plus
CrossBay Medical, Inc., a health technology company focused on improving the delivery of women’s healthcare, announced it has received clearance from the Food and Drug Administration (FDA) to market its CrossGlide ETS Plus. Similar to the CrossGlide ETS, Endometrial Tissue Sampler (which received FDA clearance this summer), the ETS Plus also enables medical providers to perform…
Read MoreCartiHeal Receives FDA “Breakthrough Device Designation” for the novel Agili-C Implant
CartiHeal, developer of Agili-C, a proprietary implant for the treatment of cartilage lesions in arthritic and non-arthritic joints, announced today that FDA has granted Breakthrough Device Designation for the Agili-C implant. FDA’s Breakthrough Device Program is reserved for certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases…
Read MoreSteris to acquire Key Surgical for $850 Million
STERIS plc (NYSE: STE) (“STERIS” or the “Company”) today announced that the Company has signed a definitive agreement to purchase Key Surgical, a portfolio company of Water Street Healthcare Partners, LLC, through a U.S. subsidiary for $850 million. STERIS anticipates that the acquisition will qualify for a tax benefit related to tax deductible goodwill. Adjusting for the present value of…
Read More