Diagnostics & Healthcare News
FDA Clears Dexcom’s First Over-the-Counter Continuous Glucose Monitor
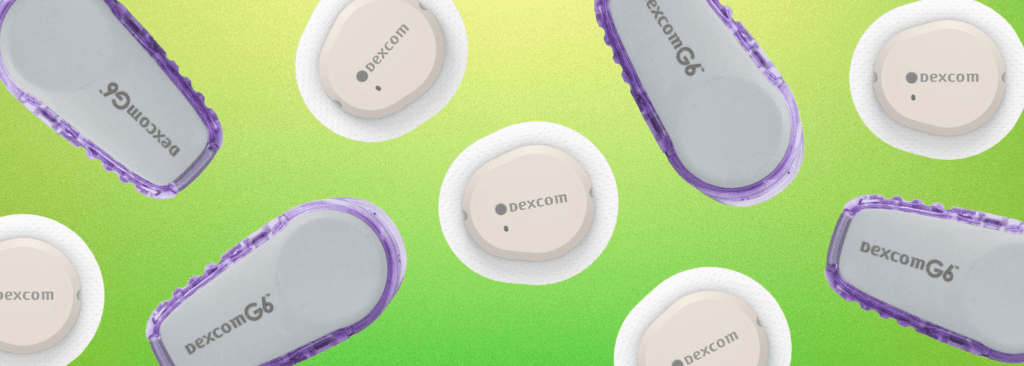
On March 5, the U.S. Food and Drug Administration (FDA) announced the clearance of Dexcom’s device, marking it as the first continuous glucose monitor available over the counter. Following this announcement, Dexcom’s shares experienced a 2.2% increase in extended trading. Known as Stelo, this device is tailored for individuals aged 18 and older who do…
Read MoreSparrow BioAcoustics Launches Software That Turns a Smartphone into a Stethoscope
Sparrow BioAcoustics recently unveiled its StethophoneTM software, which transforms smartphones into medical-grade stethoscopes. This innovative tool enables users to capture, analyze, and share crucial heart health data with medical professionals from any location. In June 2023, Sparrow received FDA clearance for the downloadable application, making it the first FDA-cleared smartphone stethoscope software-as-a-medical-device (SaMD). The company…
Read MoreEnable Injections Receives First U.S. Food and Drug Administration (FDA) Approval
Enable Injections, Inc. (“Enable”) today announced that the U.S. Food and Drug Administration (FDA) has approved the EMPAVELI Injector (enFuse®) for the subcutaneous delivery of EMPAVELI® (pegcetacoplan), which is commercialized in the United States by Apellis Pharmaceuticals, Inc. for adults with paroxysmal nocturnal hemoglobinuria (PNH). The EMPAVELI Injector is a compact, wearable injector designed to streamline the self-administration experience with minimal…
Read MoreBeacon Biosignals Receives FDA Clearance for AI-Assisted Sleep Monitoring Device Dreem 3S
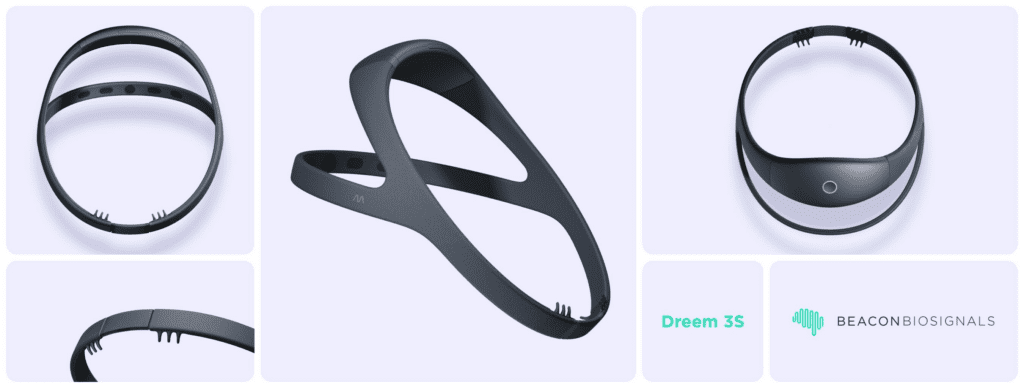
Beacon Biosignals is thrilled to announce the milestone achievement of FDA 510(k) Clearance for the Dreem 3S, an advanced wearable headband with integrated machine learning algorithms to capture electroencephalogram (EEG) data from the brain to monitor sleep architecture and aid in the diagnosis of disturbed sleep. FDA Clearance marks it as equivalent to in-lab polysomnography for the…
Read Morealveoair® has received U.S. FDA clearance, marking a significant advancement in respiratory care
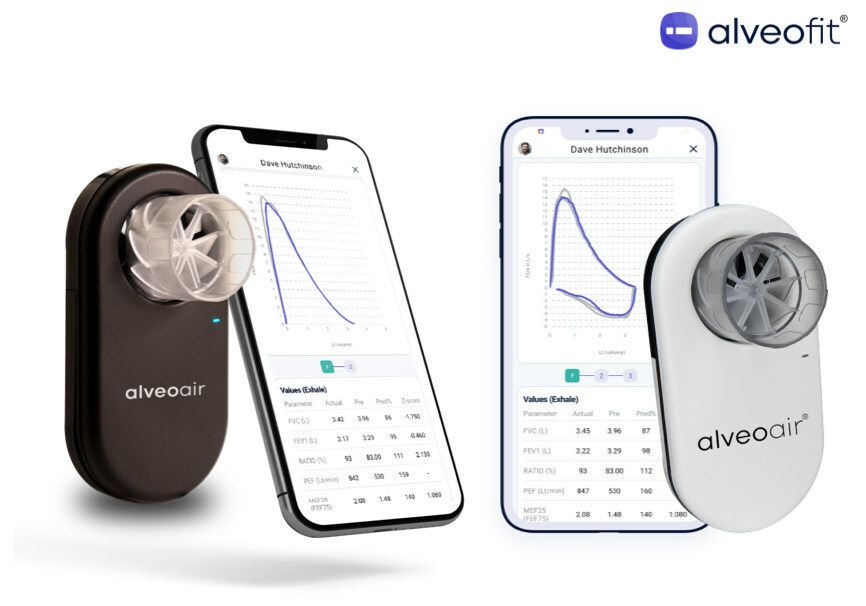
alveofit® (Roundworks Technologies Private Limited) is thrilled to announce that its groundbreaking product, alveoair® spirometer, has earned U.S. FDA clearance for distribution in the United States. Specializing in digital therapeutics for respiratory care, the company is committed to delivering affordable and interoperable lung health solutions. This U.S. FDA approval, coupled with strategic partnerships like the one…
Read MoreAbbott to Acquire Bigfoot Biomedical, Furthering Efforts to Develop Personalized, Connected Solutions for People with Diabetes
Abbott (NYSE: ABT) and Bigfoot Biomedical today announced a definitive agreement for Abbott to acquire Bigfoot, a leader in developing smart insulin management systems for people with diabetes. The transaction is subject to customary closing conditions and is expected to close in the third quarter of 2023. Financial terms were not disclosed. Abbott and Bigfoot have worked together on connected diabetes solutions since…
Read MoreParagonix Technologies Receives FDA Clearance for BAROguard™ Donor Lung Preservation System
Paragonix Technologies, a leading organ transplant company, received US Food and Drug Administration (“FDA”) clearance for its next-generation donor lung preservation system, BAROguard™. The BAROguard™ System combines Paragonix’s existing advanced hypothermic preservation technology with automated continuous and active airway pressure control, ensuring that an optimal temperature range and a clinically recommended inflation pressure range for…
Read MoreInBody Hits Milestone: 100 Million Tests Recorded Globally on Their LookinBody Web Platform
Highlighting a growing interest in body composition data, InBody is celebrating a long-anticipated milestone: 100 million tests taken globally on their body composition analyzers as of Friday, Aug. 4. “We’ve come a long way since InBody’s founding in 1996,” said Harry Yun, CEO of InBody USA. “The popularity of our tests is skyrocketing. Even the…
Read MoreWelldoc Receives 10th 510(k) Clearance from FDA for Award-Winning Diabetes Platform BlueStar®
Welldoc®, a digital health leader revolutionizing chronic care, today announced the receipt of its 10th 510(k) clearance from the Food and Drug Administration (FDA) for its award-winning diabetes digital health solution, BlueStar®. This clearance reinforces Welldoc’s position as a leader in technology in diabetes management. This 10th 510(k) clearance enables BlueStar to use connected insulin dosing…
Read MoreCeribell Receives FDA 510(k) Clearance and CMS NTAP Reimbursement for New ClarityPro™ Software with Electrographic Status Epilepticus Diagnostic Indication
Ceribell, Inc.® announced that its new software ClarityPro has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the indication of diagnosing Electrographic Status Epilepticus (ESE). The clearance follows prior receipt of Breakthrough Device Designation from FDA. Subsequent to receiving Breakthrough Device Designation and 510(k) clearance from FDA, the U.S. Centers for Medicare…
Read More