Archive for June 2022
Centerline Biomedical Raises $33 Million in Series B Financing
Centerline Biomedical, Inc. (Centerline), a private medical technology company, announced the closing of a $33 million Series B equity financing led by Cleveland Clinic with participation by GE Healthcare, RIK Enterprises, JobsOhio, Jumpstart Ventures and G2 Group Ventures. This funding will help propel the company into new surgical applications, accelerate its commercial sales and add to its…
Read More4 Easy Ways to be a Prepared Passive Candidate
Typically, career advice is written for the Job Seeker, and rarely for the gainfully employed. Likewise, it is much easier (and more ideal) to find a new position while employed, and similarly, it is much easier to build an enticing personal brand and professional portfolio while actively engaged in a career. So, for those who…
Read MoreUse Your Internship to Network for Your Career
One of the most sought after experiences for many college students is to partake in an internship applicable to their studies. An internship can be extremely valuable, and an important aspect of an internship position is the significant opportunity to network with professionals already working in a particular field (…not to mention their field of…
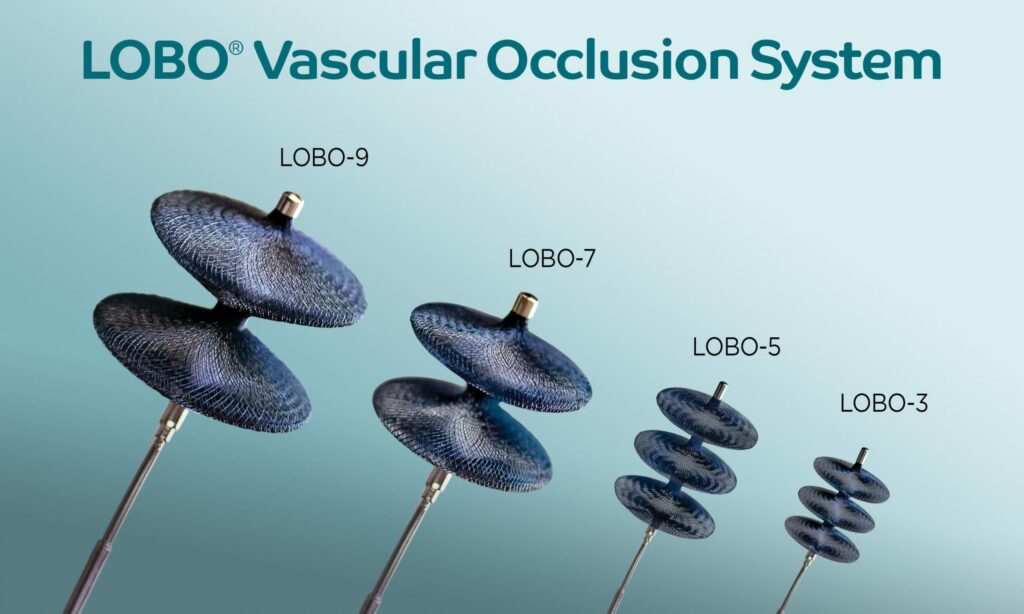
Read MoreOkami Medical Announces FDA 510(k) Clearance of the LOBO-7 and LOBO-9 Vascular Occluders to Address a Wide Range of Peripheral Embolization Cases
Okami Medical Inc., a medical device company focused on developing innovative solutions that address key unmet clinical needs in peripheral vascular intervention, announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the LOBO-7 and LOBO-9 Vascular Occluders, the latest addition to the company’s LOBO® Vascular Occlusion System. The LOBO (LOw-profile Braided Occluder) system, purpose-built for…
Read MoreXenco Medical Expands its Ambulatory Surgery Center Device Portfolio with FDA Clearance and Launch of its Multilevel CerviKit
Xenco Medical has expanded its ASC surgical device portfolio through the FDA clearance and launch of its Multilevel CerviKit™, an expansion of Xenco Medical’s breakthrough single-use cervical spine technology to include a comprehensive suite of implants and single-use instruments for 2, 3, and 4 level anterior cervical spine procedures. Optimized for the ambulatory surgery center…
Read More7 Career Mistakes To Avoid
“The only one managing your career is you!” You’ve probably heard this advice many times from multiple sources. However, these well-meaning advisors may have neglected to tell you that managing your career is hard! You spent the formative years of your life and education following a prescribed plan with a mapped-out path to success. However,…
Read MoreSaneso Novel 360° Colonoscope Receives FDA Clearance
Saneso Inc., a leading US developer of next gen endoscope systems announces today the US Food and Drug Administration (FDA) has granted 510(k) clearance of its novel integrated 360° field of view colonoscope. “We are pleased to extend the world’s first and only 360° colonoscope to all physicians, hospitals, and ASCs who will benefit from…
Read MoreCardio Flow, Inc., Announces FDA Clearance for FreedomFlow® Peripheral Guidewire, and their First Commercial Case Completed in U.S.
Cardio Flow, Inc., a medical device company and developer of minimally invasive peripheral vascular devices to treat peripheral artery disease (PAD), announced it recently received U.S. Food and Drug Administration (FDA) clearance for the company’s FreedomFlow® Peripheral Guidewire. The FreedomFlow guidewire is stainless steel core-to-tip design with a fixed distal-spring coil which was developed to…
Read More