Archive for January 2019
Stryker Installed an Impressive 54 Mako Robots in Q4 2018
Stryker made the market wait for more than a year after FDA approval for the full commercial launch of its Mako total knee product, prompting us to wonder, at the time, if the robot would be worth the wait. It’s safe to say, after seeing the company’s latest earnings report, that the answer is an unequivocal…
Read MoreFDA Breaks Record for Medical Device Approvals in 2018
It’s an unprecedented time for innovation and regulation in medical devices. The Food and Drug Administration announced that 2018 was their most successful year in approving medical devices. 106 devices received the FDA’s marketing green light, breaking the record of 99 in 2017. These devices included automated insulin dosing systems for children as young as 7, the world’s…
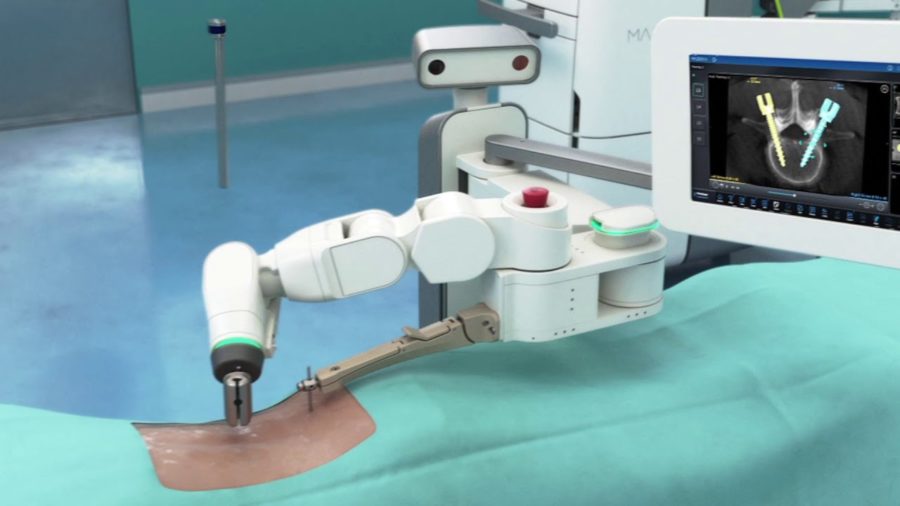
Read MoreMedtronic Announces First U.S. Spine Patients Treated with Mazor X Stealth Robotic Surgical System
Medtronic today announced the first U.S. patients treated with the Mazor X Stealth Edition for spine surgery following its recent commercial launch. The Mazor X Stealth Edition offers a fully-integrated procedural solution for surgical planning, workflow, execution and confirmation. The system was first used at Norton Healthcare in Louisville, Ky., and Reston Hospital Center in…
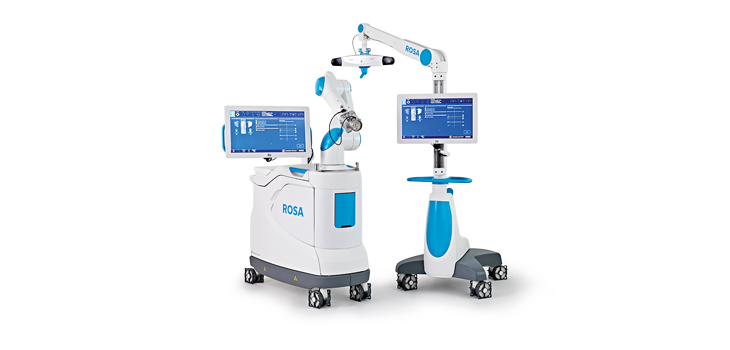
Read MoreZimmer Biomet Receives FDA Clearance for ROSA® Knee System for Robotically-Assisted Surgeries
Zimmer Biomet Holdings, Inc., a global leader in musculoskeletal healthcare, today announced U.S. Food and Drug Administration 510(k) clearance of the ROSA® Knee System for robotically-assisted total knee replacement surgeries. ROSA Knee features 3D pre-operative planning tools and real-time, intraoperative data on soft-tissue and bone anatomy designed to improve bone cut accuracy and range of motion…
Read MoreOrchid Orthopedic Solutions Acquired by European Private Equity Firm
Nordic Capital Fund IX announced today that it has agreed to acquire a majority holding in Orchid Orthopedic Solutions, from Altor Fund III who will retain a significant minority holding in the company. Headquartered in Holt, Michigan, Orchid is a world leader in the design and manufacture of implants to the global orthopedic market. Orchid’s leading…
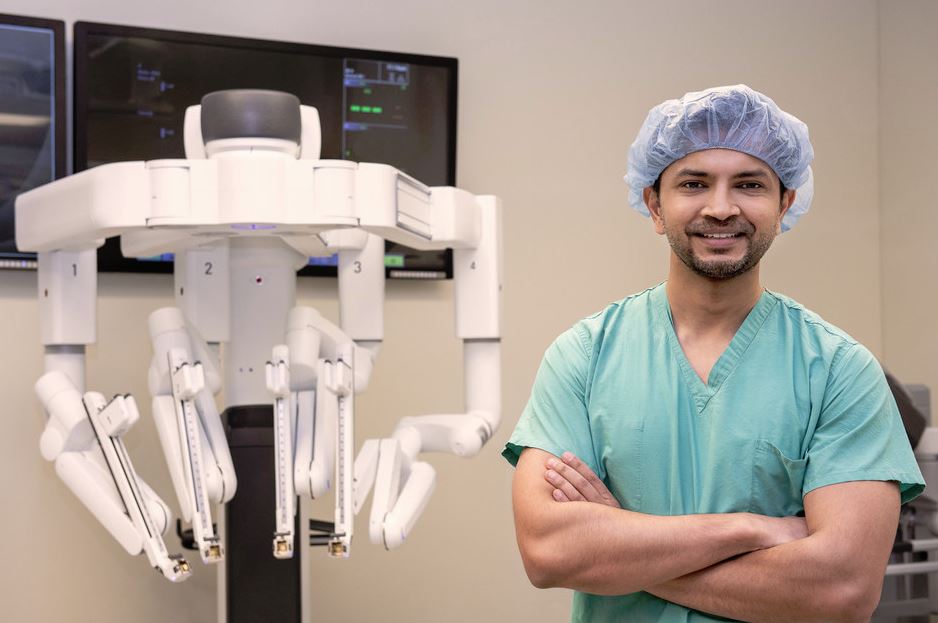
Read MoreNorthwestern Memorial Hospital Performs First US Robotic Lung Volume Reduction Surgery
Northwestern Memorial Hospital is the first in the country to successfully complete robotic-assisted lung volume reduction surgery (LVRS) using the da Vinci Xi Surgical System® to remove diseased, emphysematous tissue within the lungs for the treatment of severe emphysema, a progressive form of chronic obstructive pulmonary disease (COPD). The robotic procedure allows the surgeon to precisely remove the…
Read More1 in 3 GoFundMe Campaigns are for Medical Bills and That’s a Problem Says GoFundMe CEO
Scrolling through the GoFundMe website reveals seemingly an endless number of people who need help or community support. A common theme: the cost of health care. It didn’t start out this way. Back in 2010, when the crowdfunding website began, it suggested fundraisers for “ideas and dreams,” “wedding donations and honeymoon registry” or “special occasions.”…
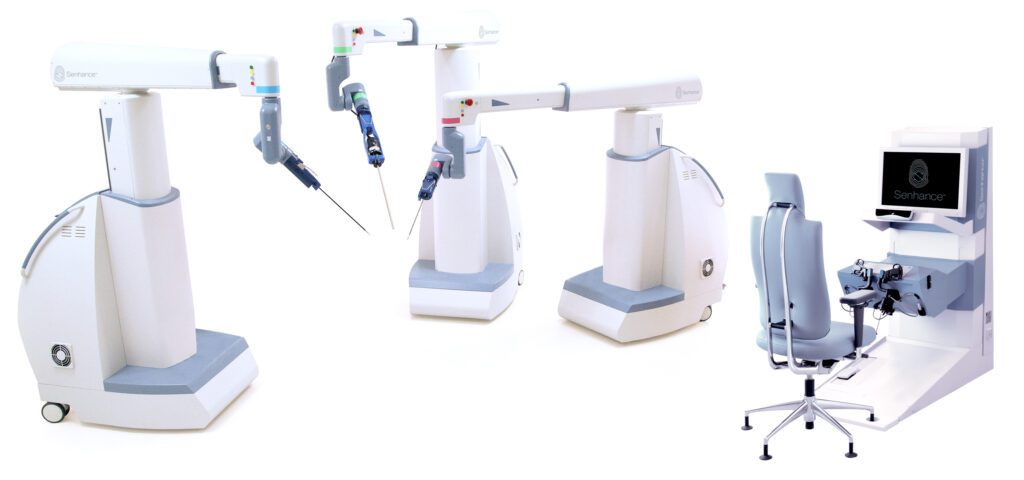
Read MoreTransEnterix Wins FDA Clearance for Senhance Ultrasonic Instruments
TransEnterix, Inc., a medical device company that is digitizing the interface between surgeons and patients to improve minimally invasive surgery, today announced the Company received FDA 510(k) clearance for its Senhance Ultrasonic System. “Advanced energy devices are used within a high percentage of cases across a wide range of procedures, which make them a critical tool for…
Read MoreOSSIO Receives 501(k) Clearance for Bio-Integrative Bone Pins
The FDA has granted 510(k) clearance to Ossio for its family of bio-integrative bone pins, which secure broken bones during the healing process while leaving no permanent hardware behind. The pins are made of a natural mineral fiber matrix that aims to replace the more permanent, metal fixation implants used in fractures, bone cuttings or…
Read MoreIRRAS’s Intracranial Bleeding Management Device Used on First U.S. Patient
IRRAS AB, a commercial-stage medical-technology company, today announced that the first patient in the United States was successfully treated with IRRAflow, the company’s initial product that offers an innovative, therapeutic approach to the fluid management of patients with intracranial bleeding. “The US is the largest market for intracranial procedures, and we are making significant progress engaging neurocritical care centers and specialty hospitals across the country,” said Kleanthis G. Xanthopoulos, Ph.D.,…
Read More