Archive for September 2020
Smith+Nephew Acquires Integra Lifescience’s Extremity Orthopedics Business for $240
Smith+Nephew, the global medical technology business, announces that it has agreed to acquire the Extremity Orthopaedics business of Integra LifeSciences Holdings Corporation for $240 million. The acquisition supports Smith+Nephew’s strategy to invest in higher-growth segments. This acquisition will significantly strengthen Smith+Nephew’s extremities business by adding a combination of a focused sales channel, complementary shoulder replacement and upper…
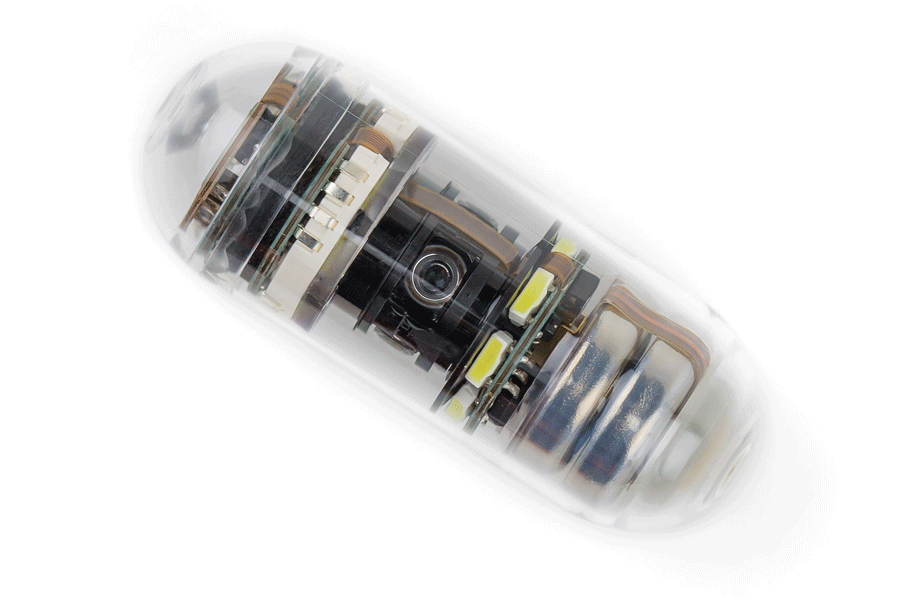
Read MoreFDA Allows At-Home Administration for CapsoVision Capsule Endoscope
CapsoVision, an innovator in the gastroenterology diagnostics market, today announced that the U.S Food & Drug Administration (FDA) will apply enforcement discretion which allows at-home administration of the CapsoCam Plus® small bowel capsule endoscope during the COVID-19 pandemic for patients who are determined eligible for at-home administration. The labeling addendum permits a fully remote capsule endoscopy…
Read MoreNOUS Imaging Receives 510(k) Clearance from FDA for Real Time MRI Software
NOUS Imaging, a medical imaging software company, today announced that the FDA has cleared its 510(k) submission for FIRMM, Framewise Integrated Real-Time MRI Monitoring. FIRMM provides real-time monitoring and biofeedback that addresses the significant problem of patient motion during brain MRI. This is the Company’s first FDA clearance and represents the initial piece of a…
Read MoreTHINK Surgical and Total Joint Orthopedics Announce Collaboration on Robotic Knee Surgery
Total Joint Orthopedics (TJO) announced today a new collaboration with THINK Surgical®, Inc., an advanced orthopedic robot technology company based in Fremont, CA. Under the terms of the agreement, THINK Surgical will develop software allowing TJO’s Klassic® Knee System to be implanted using THINK’s TSolution One® Total Knee Application and both companies will co-market the solution upon…
Read MoreProstate Cancer Protection Company BioProtect Raises $25M
BioProtect ltd., a private company with a unique bioabsorbable polymer spacer balloon platform, announced the final closing of $25 million of its Series D equity financing from an unnamed strategic investor and Peregrine Ventures. Every year, more than 1.1 million men are diagnosed with prostate cancer worldwide and approximately 400,000 will undergo prostate radiotherapy. Historically prostate radiation…
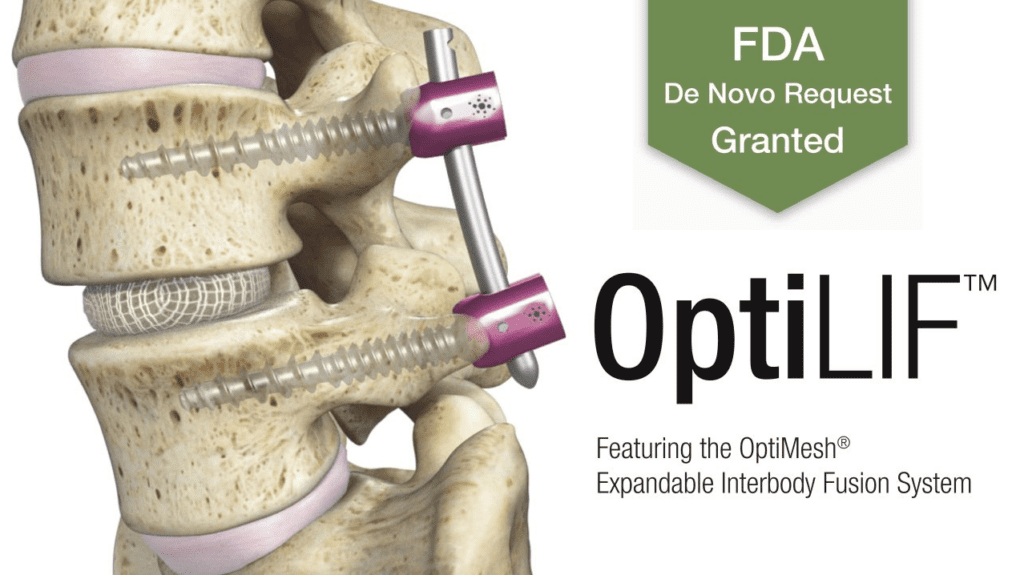
Read MoreSpineology Gets FDA De Novo Grant of Minimally Invasive OptiMesh Expandable Interbody Fusion System
Spineology Inc., an innovator in anatomy-conserving surgery, is excited to announce the FDA grant of its proprietary Spineology Interbody Fusion System, now called the OptiMesh Expandable Interbody Fusion System. The grant follows the successful completion of the SCOUT (Spineology Clinical Outcomes Trial) Investigational Device Exemption (IDE) trial. OptiMesh is a unique mesh device that expands…
Read MoreCerapedics Announces Canadian Approval of its Next-generation Bone Graft
Cerapedics, a private ortho-biologics company, today announced the Health Canada approval of the company’s next-generation bone graft called i-FACTOR+ Matrix, making it the first market to approve the commercial launch of this product. “We are excited to announce Canadian regulatory approval of our next-generation product, based on P15 osteogenic cell-binding peptide , i-FACTOR+ Matrix,” said…
Read MoreIntersect ENT Announces Agreement to Acquire Fiagon for $71M
Intersect ENT, Inc., a company transforming care for patients with ear, nose and throat (“ENT”) conditions, today announced it has entered into an agreement to acquire Fiagon AG Medical Technologies, a leader in electromagnetic surgical navigation solutions, for €60 million in cash. Under the terms of the transaction, Intersect ENT will make an initial €15 million payment at…
Read MoreFitbit Wins FDA 510(k) Clearance and CE Mark for ECG App
Fitbit has received 510(k) clearance from the U.S. Food and Drug Administration (FDA), as well as Conformité Européenne (CE)marking in the European Union, for its electrocardiogram (ECG) app to assess heart rhythm for atrial fibrillation (AFib), a condition that affects more than 33.5 million people globally. The Fitbit ECG App, unveiled in Fitbit’s recent fall product launch, will…
Read MoreAvinger Receives FDA Clearance of Ocelaris Next Generation Image-guided CTO Crossing System
Avinger, Inc., a commercial-stage medical device company marketing the first and only intravascular image-guided, catheter-based system for diagnosis and treatment of patients with Peripheral Artery Disease (PAD), today announced that the Company received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for its Ocelaris next generation image-guided chronic total occlusion (CTO) crossing system.…
Read More