Archive for March 2019
Itamar Medical Launches Next-Generation WatchPAT System for Home Sleep Apnea Testing
Itamar Medical Ltd., a company that develops, manufactures and markets non-invasive diagnostic medical devices for sleep apnea with a focus on the cardiology market, today announced the launch of WatchPAT 300, the next generation WatchPAT system for home sleep apnea testing. WatchPAT 300 includes several advances that are designed to enhance both patients’ WatchPAT experience…
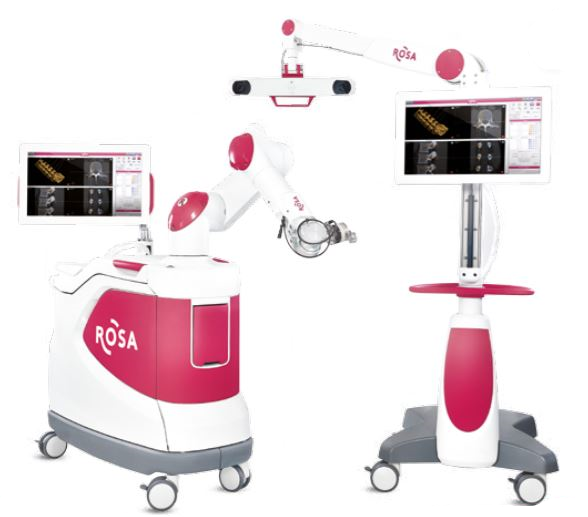
Read MoreZimmer Biomet Receives FDA Clearance for Robotic ROSA One Spine System
Zimmer Biomet Holdings, Inc., a global leader in musculoskeletal healthcare, today announced U.S. Food and Drug Administration 510(k) clearance of the ROSA® ONE Spine System for robotically assisted minimally invasive and complex spine surgeries, strengthening the Company’s comprehensive ROSA® ONE Brain and ROSA® Knee portfolio. ROSA ONE Spine combines robotics and navigation while delivering a unique real-time patient…
Read MoreFDA Approves Breakthrough Optimizer Smart Device for Chronic Heart Failure
Impulse Dynamics, developer of the implantable Optimizer® Smart System for delivering CCM™ therapy, announced today that it has received approval from the United States Food and Drug Administration for its first-in-class Optimizer Smart System (link to FDA announcement: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634103.htm). Designed to address a significant unmet medical need in heart failure, the Optimizer Smart System received Breakthrough…
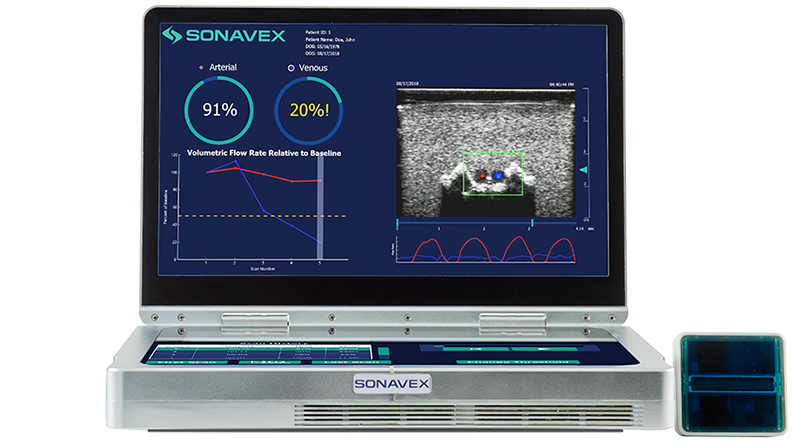
Read MoreSonavex Receives FDA Clearance for Blood Flow Monitoring Technology
Following peripheral vascular and microvascular surgeries, it is important to be able to monitor how blood is flowing through the treated vessels and whether there may be an occlusion or compromise. This monitoring typically requires a nurse or a trained sonographer to use Doppler ultrasound, but now a new option is available. Sonavex, a company…
Read MoreStryker Acquires Rotator Cuff Device Maker OrthoSpace
Stryker announced today it has completed the acquisition of OrthoSpace, Ltd., a privately held company founded in 2009 and headquartered in Caesarea, Israel, in an all cash transaction for an upfront payment of $110 million and future milestone payments of up to an additional $110 million. OrthoSpace’s product portfolio provides a highly differentiated technology for…
Read MoreSmith & Nephew to Buy Osiris Therapeutics for $660 Million
Smith & Nephew said today that it agreed to put $660 million on the table to acquire Osiris Therapeutics and its regenerative medicine portfolio. The British orthopedics and wound care giant said the $19-per-share deal is a 37% premium on the 90-day volume-weighted average for OSIR shares. It’s structured as a two-step tender offer, Smith & Nephew said,…
Read MoreSaranas Achieves De Novo FDA Clearance for Their Endovascular Monitoring System
Saranas Inc., a medical device company with innovative technology for real-time detection and monitoring of internal bleeding during endovascular procedures, today announced that it has been granted de novo classification by the U.S. Food and Drug Administration (FDA) for the Early Bird Bleed Monitoring System. According to a recent study of over 17,000 large-bore transcatheter…
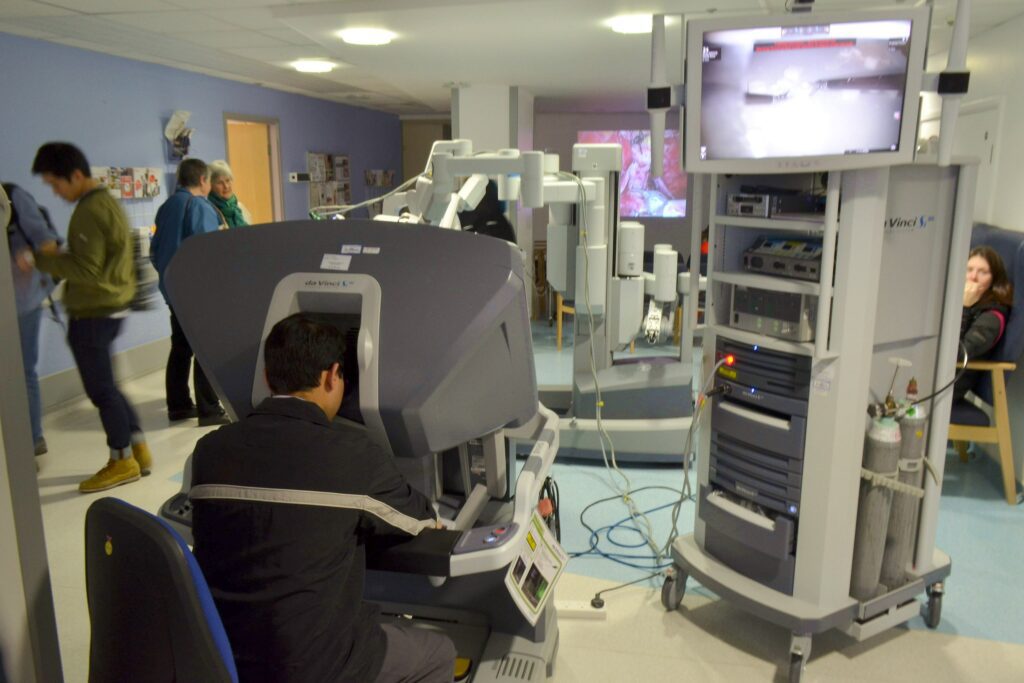
Read MoreFDA Warns Against Using Surgical Robots in Cancer Surgeries
The Food and Drug Administration on Thursday warned against the use of robotically assisted devices for mastectomies and other cancer surgeries, asserting the products may pose safety risks and result in poor outcomes for patients. The agency said it decided to issue its warning after reviewing studies suggesting that robotically assisted devices were being used to perform…
Read More