Archive for April 2019
First Medical Device Treatment for Childhood ADHD Cleared by FDA
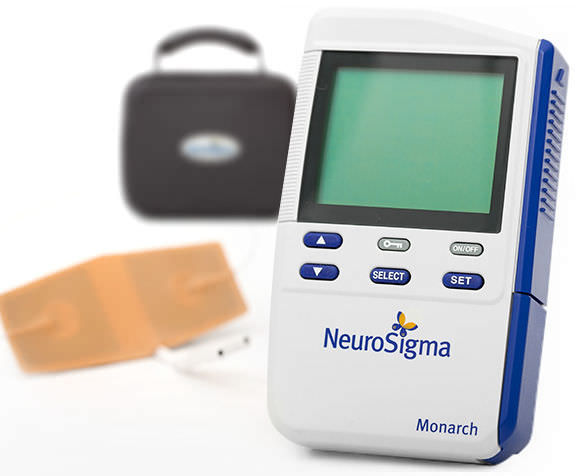
NeuroSigma won de novo clearance from the FDA last week to put the first medical device for treating attention-deficit hyperactivity disorder on the U.S. market. Los Angeles-based NeuroSigma makes the Monarch eTNS system, which is designed to deliver mild electrical signals through a forehead patch to stimulate branches of the trigeminal nerve during sleep. The device won CE Mark approval…
Read MoreExactech Announces First Successful Surgeries Using InterSep Bone Void Filler
Exactech, a developer and producer of bone and joint restoration products for extremities, hip, and knee, announced today the successful first surgery using its new InterSepTM calcium sulfate bone void filler. One of the newest additions to Exactech’s infection-related solutions, InterSep is a 100% synthetic calcium sulfate bone void filler that is engineered to fully resorb and…
Read MoreElucent Medical Wins FDA Clearance for New Breast Surgery Navigation System
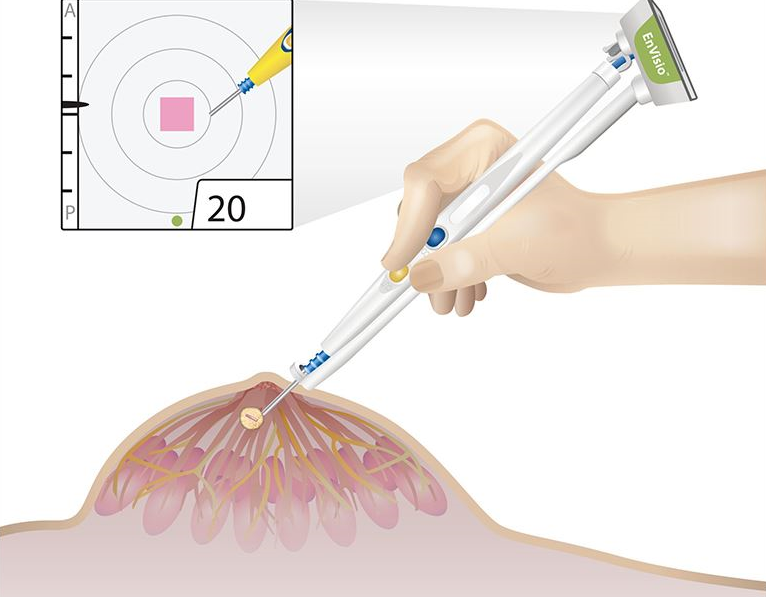
Elucent Medical, a company dedicated to developing better pathways for breast cancer care, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the clinical use of the company’s EnVisio™ Surgical Navigation System. Placed at the time of the breast biopsy, Elucent Medical’s permanently implantable wireless SmartClip™ Soft Tissue Marker…
Read MoreFirst-Ever Clinical Trial Initiated for Neural Interface that Turns Thoughts into Speech
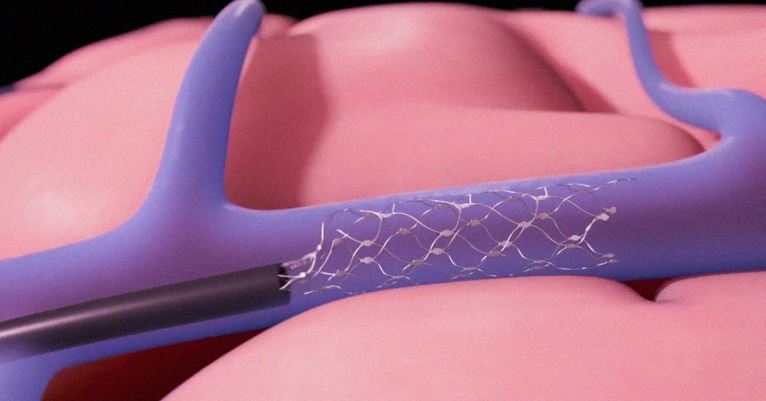
Synchron, Inc. today announced the initiation of the first clinical trial for the Stentrode™, a minimally-invasive neural interface technology being investigated for restoration of communication in people with severe paralysis. The trial will evaluate the safety of Thought-to-Text™ technology in patients, by assessing the Stentrode implant in combination with BrainOS™ software. The Stentrode is designed to record brain activity…
Read MoreFDA Approves First New Therapy to Treat Heart Attacks in Years
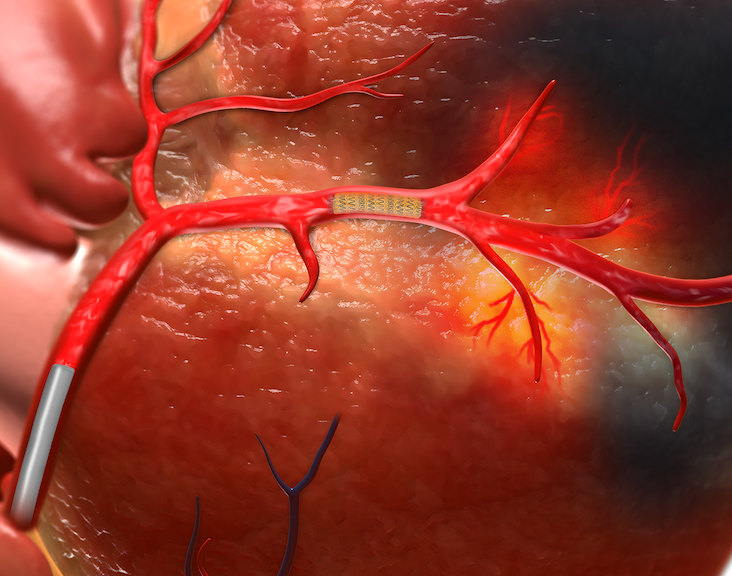
TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) granted premarket approval for its SuperSaturated Oxygen (SSO2) Therapy. SSO2 Therapy provides interventional cardiologists with the first and only FDA-approved treatment beyond percutaneous coronary intervention (PCI) to significantly reduce muscle damage in heart attack patients. SSO2 Therapy delivers hyperbaric levels of oxygen directly to the ischemic…
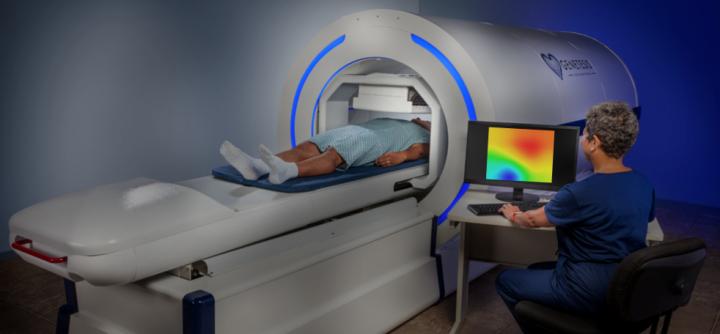
Read MoreGenetesis CardioFlux Platform Receives FDA 510(k) Clearance
Genetesis, a company based in Mason, Ohio, won FDA clearance for its cardiac imaging offering that combines the CardioFlux magnetocardiograph with the the integrated Faraday Analytical Cloud (FAC). The technology is intended to be used by ER physicians and cardiologists to quickly assess patients presenting with chest pain, helping with triage and getting patients to receive…
Read More