Archive for March 2023
Positive Results From PROMISE II Pivotal Trial Showing that LimFlow System Saves Most Patients with End-stage Peripheral Artery Disease From Major Amputation
LimFlow SA, a pioneer in the development of minimally-invasive technology for the treatment of chronic limb-threatening ischemia (CLTI), a severe form of peripheral artery disease (PAD), announced today that results from the PROMISE II U.S. pivotal trial were published in today’s issue of the New England Journal of Medicine (NEJM). The results demonstrated that the…
Read MoreAd Astra Diagnostics Files 510(k) for QScout™ Hematology Analyzer
Ad Astra Diagnostics (AAD), developer of rapid diagnostic systems, announced that it has submitted a 510(k) application to the U.S. Food and Drug Administration (FDA) for its QScout RLD rapid-result hematology system intended to report white blood cell count, neutrophil-to-lymphocyte ratio, and counts of six white blood cell types in fingerstick or venous blood. QScout is the…
Read MoreHow Office Buzzwords are Hurting Your Communication, and How to Fix it
Let’s do a deep dive on best practices to maximize our synergy to move the needle on low-hanging fruit. Or, let’s collaborate on some processes to get started on our most attainable objectives. So, which one sounds better? We’re guessing the second option. So why are we letting our workplace communication get so…
Read MoreAdvamedica Inc announces FDA clearance for Ax-Surgi Surgical Hemostat
Advamedica Inc., a biomaterial focused medtech startup today announced the US Food and Drug Administration (FDA) 510(k) clearance for its latest innovation, Ax-Surgi Surgical Hemostat. With this, Ax-Surgi has become first and only chitosan based hemostat cleared for controlling severe surgical bleeding. Ax-Surgi is based on novel biopolymer platform and controls bleeding through its bioadhesive…
Read MoreFemDx Medsystems, Inc. Receives FDA Clearance For Its FalloView™ Falloposcope
FemDx Medsystems, Inc., a women’s health startup based in Santa Clara, CA announces the company has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its FalloView™ device. According to Ashlee Francis, CEO of FemDx Medsystems, Inc., the device is the first totally disposable 1.2mm diameter falloposcope incorporating a CMOS chip endoscope to evaluate…
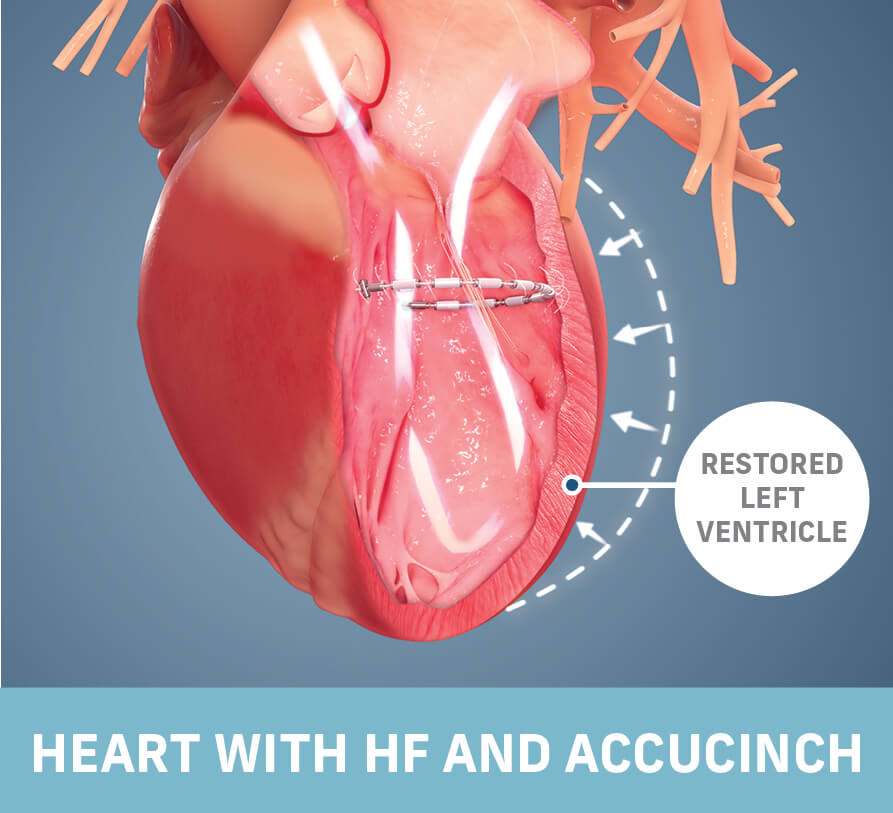
Read MoreAncora Heart’s AccuCinch System Demonstrates Significant Improvement in Quality of Life, Cardiac Structure and Function in Heart Failure Patients
Ancora Heart, Inc., a company developing a completely transcatheter device-based therapy to address heart failure (HF), announced that patients treated with the investigational AccuCinch® Ventricular Restoration System demonstrated improvement in HF patient outcomes and beneficial changes in the structure of the heart. The 12-month data were presented as part of a late-breaking clinical science session at…
Read MoreFDA Expands Indications for Use of FibroScan® for Comprehensive Liver Management
The U.S. Food and Drug Administration (FDA) has cleared expanded Indications for Use for screening with FibroScan®, the non-invasive liver management technology by Echosens. The update furthers FibroScan® accessibility to more people, enabling physicians to identify those at risk of suffering adverse liver outcomes and connecting them with preventative care and treatment options. The new clearance removes…
Read MoreLumicell Submits New Drug Application for LUMISIGHT™ Optical Imaging Agent to U.S. FDA for Intraoperative Breast Cancer Detection and Removal
Lumicell, Inc., a privately held company focused on innovative fluorescence-guided imaging technologies for cancer surgery, today announced a New Drug Application (NDA) for its LUMISIGHT™ Optical Imaging Agent has been submitted to the U.S. Food and Drug Administration (FDA). LUMISIGHT is intended for use with the Lumicell™ Direct Visualization System (DVS), an investigational system designed…
Read MoreAsensus Surgical Receives FDA 510(k) Clearance For Pediatric Indication for Senhance Surgical System
Asensus Surgical, Inc. (NYSE American: ASXC), a medical device company that is digitizing the interface between the surgeon and the patient to pioneer a new era of Performance-Guided Surgery™ (PGS), announced that it has received 510(k) clearance from the FDA for an expanded indication to treat pediatric patients with the Senhance® System. The Senhance System…
Read MoreDermaSensor Unveils Study Results Demonstrating Ability to Detect Skin Cancer With Device Granted FDA Breakthrough Designation
DermaSensor Inc., a health technology company equipping primary care physicians (PCPs) with a non-invasive device for use in evaluation of suspicious skin lesions, presented a pooled analysis today from two clinical studies evaluating the performance of the company’s device in detecting skin cancer. In the DERM-ASSESS III and DERM-SUCCESS clinical studies, the device had a…
Read More