Archive for March 2023
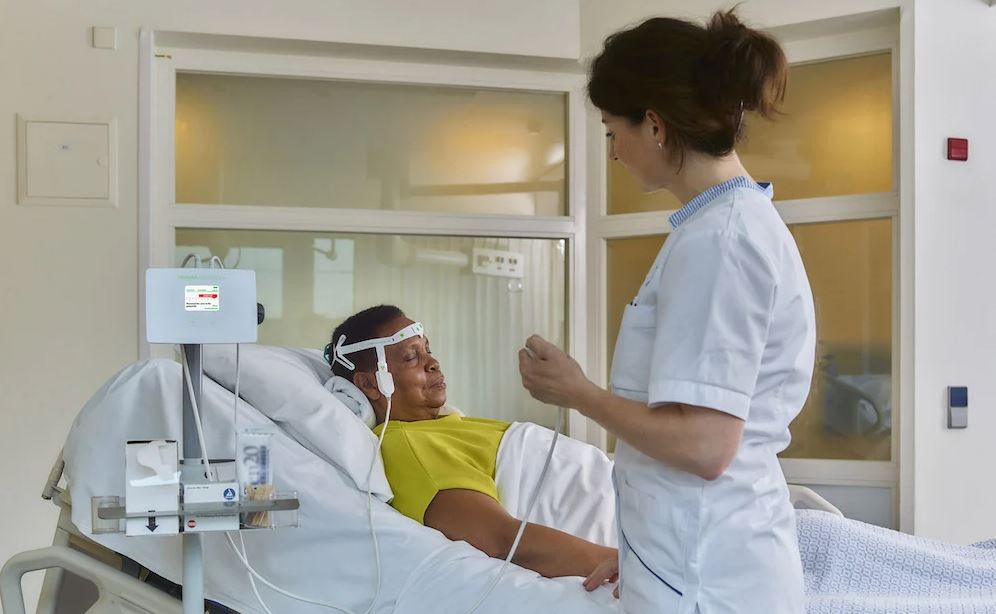
Prolira Announces FDA Clearance of the DeltaScan® Brain State Monitor for Detecting Acute Encephalopathy (Acute Brain Failure), a Condition Affecting Millions of Hospitalized Patients 60 and Older
Prolira BV, a high-tech company committed to enabling early and effective treatment of patients at risk of developing acute brain failure, announced it has received 510(k) clearance from the Food and Drug Administration (FDA) for the DeltaScan® Brain State Monitor to aid in the diagnosis of acute encephalopathy in hospitalized patients over 60 years of…
Read MoreHeartBeam Announces Acquisition of LIVMOR Assets
HeartBeam, Inc. (NASDAQ: BEAT), a cardiac technology company that has developed the first and only 3D-vector electrocardiogram (VECG) platform for heart attack detection anytime, anywhere, announced the strategic acquisition of substantially all assets from LIVMOR, a digital health solutions company providing a patient-engaging remote monitoring system of critical physiological biomarkers. The acquisition extends HeartBeam’s reach in…
Read MoreNanoVibronix Announces Positive Results from Independent Product Trial of UroShield for Patients with a Spinal Cord Injury
NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company’s proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced the positive evaluation results for its UroShield device, presented at a recent medical conference by clinicians from the Royal National Orthopaedic Hospital (“RNOH”). The report concluded that the Company’s UroShield…
Read MoreVivasure Medical Announces FDA IDE Approval to Initiate U.S. Pivotal Study
Vivasure Medical® Limited (“Vivasure” or the “Company”), a company pioneering novel fully absorbable technology for percutaneous vessel closure, announced that the U.S. Food and Drug Administration (FDA) has granted an Investigational Device Exemption (IDE) to advance the company’s PATCH Clinical Study, a multi-center, single-arm, pivotal study evaluating the safety and effectiveness of the Vivasure PerQseal® Closure Device…
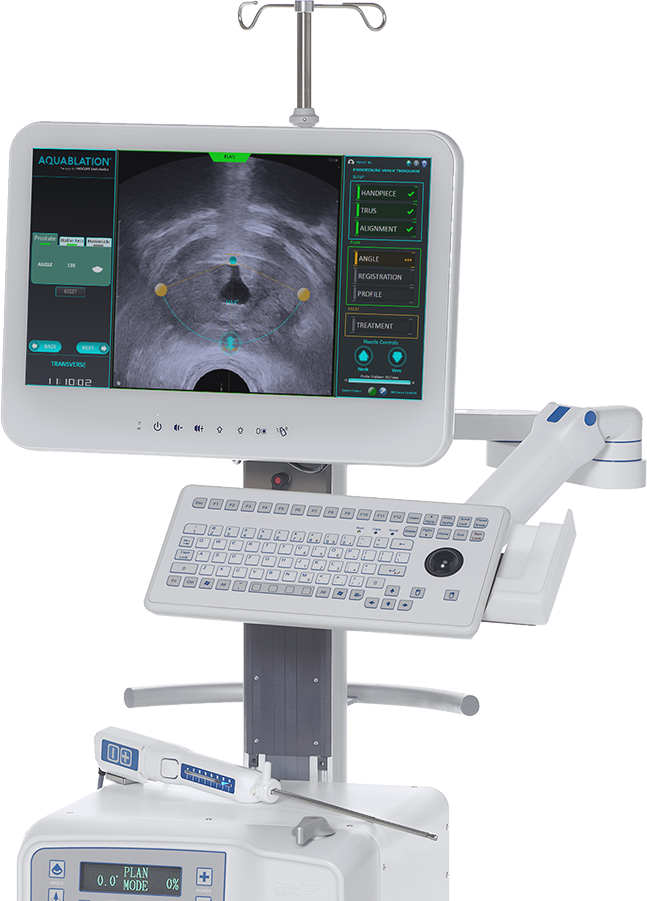
Read MoreAquablation Therapy is the first surgical robotics system to receive a MedTech Innovation Briefing (MIB) from the National Institute for Health Care Excellence
PROCEPT® BioRobotics Corporation today announced Aquablation Therapy received a MedTech Innovation Briefing (MIB) from the National Institute for Health Care Excellence (NICE), for benign prostate hyperplasia (BPH) in the United Kingdom. NICE has recognized Aquablation Therapy is as effective as transurethral resection of the prostate (TURP) for the removal of prostate tissue for men with BPH. A panel of…
Read MoreX-Bolt Orthopedics Announces FDA 510(k) Clearance for Pro-X1™ Trochanteric Nail
X-Bolt, an emerging innovator of orthopedic devices, is pleased to announce that its hip fracture solution, the Pro-X1 Trochanteric Nail, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). Pro-X1 will be X-Bolt’s flagship launch in the United States. “We are thrilled to receive FDA clearance for the Pro-X1 Trochanteric Nail,” said Brian Thornes,…
Read MoreShoulder Innovations Announces Oversubscribed $42 million Series D Financing
Shoulder Innovations Inc. (SI), a Grand Rapids, Michigan based leader in the shoulder replacement implant market, announced closing on an oversubscribed $42 million Series D financing. SI is a privately-held medical device company with a broad team of successful innovators in the shoulder space. The financing was led by Gilde Healthcare Partners, a specialized trans-Atlantic healthcare investor. US Venture Partners, Lightstone Ventures,…
Read MoreLive Remote Cardiac MRI Exam to Showcase Vista.ai’s Automated and Virtual Scan Capabilities at ACC.23/WCC
Vista.ai, a pioneer and leader in automated MRI solutions, today announced that Dr. Michael Salerno, Chief, Cardiovascular Imaging at Stanford Health Care, will conduct a live remote cardiac MRI (CMR) scan using the company’s One Click MRI™ software at the American College of Cardiology’s 72nd Annual Scientific Session Together With World Heart Federation’s World Congress…
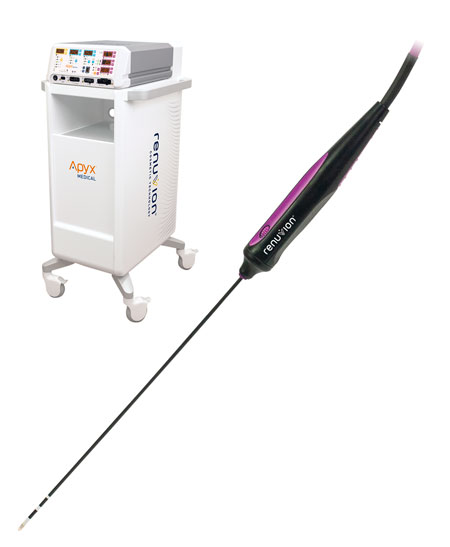
Read MoreApyx Medical Corporation Receives FDA 510(k) Clearance for the Use of Renuvion® to Coagulate and Contract Soft Tissues
Apyx Medical Corporation (NASDAQ:APYX), the manufacturer of a proprietary helium plasma and radiofrequency technology marketed and sold as Renuvion®, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for the use of the Renuvion APR Handpiece “for the delivery of radiofrequency energy and/or helium plasma where coagulation/contraction of soft…
Read MoreSpectraWAVE Secures 510(k) Clearance of HyperVue™ Intravascular Imaging System
SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for patients with coronary artery disease (CAD), today announced Food and Drug Administration (FDA) 510(k) clearance of their flagship intravascular imaging system, HyperVue™. The system combines next-generation DeepOCT™ images and near infrared spectroscopy (NIRS) with state-of-the-art ease of use to support physicians…
Read More