Surgery and Surgical Robotics
Brainomix and Nanoflex Robotics to Collaborate on an AI-Assisted Robotic System for Remote Stroke Intervention
Brainomix Ltd, an AI-powered medtech solutions company, and Nanoflex Robotics AG, a remote robotic surgical company based in Switzerland, have been awarded a grant under the “UK – Switzerland Bilateral: Collaborative R&D” program, in which the companies will work together to jointly develop an integrated remote diagnosis and treatment platform for stroke, powered by artificial intelligence.…
Read MoreCadence Announces the Acquisition of ARC Group Worldwide’s Florida Location
Cadence, Inc., a leading provider of design, development, and contract manufacturing services to the medical device, drug delivery, and diagnostics markets, announced today that it has acquired the Florida location of ARC Group Worldwide (arcw.com), a precision manufacturer specializing in Metal Injection Molding (MIM) and cleanroom plastic injection molding. ARC Florida is located in DeLand, Fla. “Acquiring ARC Florida is…
Read MoreCereVasc Receives FDA IDE Approval to Expand its Clinical Study of Patients with Normal Pressure Hydrocephalus Utilizing the Generation 2 eShunt® System
CereVasc, Inc., a clinical-stage, medical device company developing novel treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) supplement to permit the expansion of the study of the eShunt System in patients with Normal Pressure Hydrocephalus (NPH) to additional study participants and clinical sites.…
Read MoreLevita® Magnetics Wins FDA Clearance for Pioneering MARS™ System
Levita Magnetics, whose mission is to help more patients get access to better surgery, announced today it has received U.S. Food and Drug Administration (FDA) clearance for its MARS™ platform. The Levita MARS system is a first-of-its-kind minimally invasive surgical platform aimed at the high-volume abdominal surgery market. Harnessing the power of both magnets and…
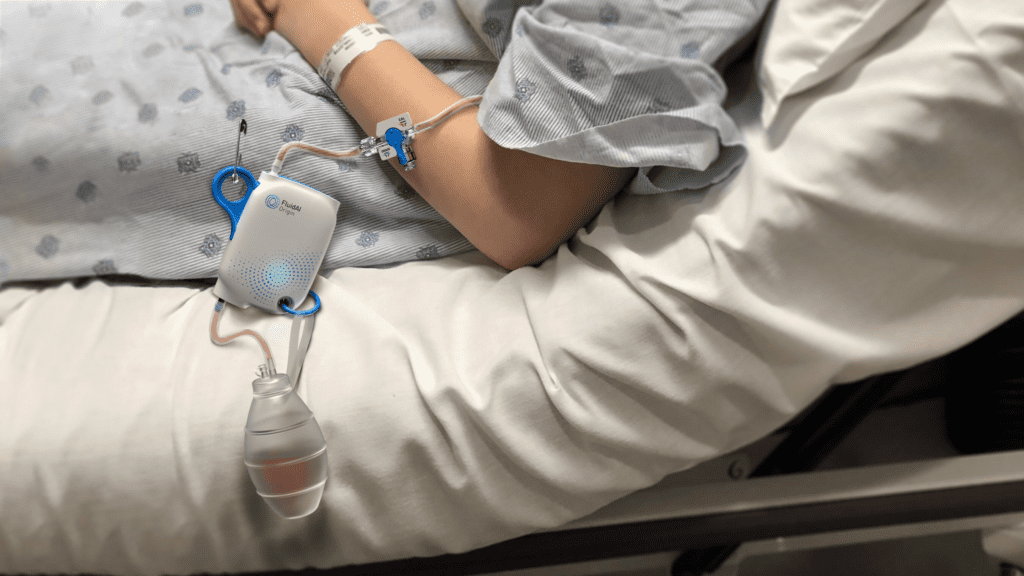
Read MoreFluidAI’s artificial intelligence-powered postsurgical monitor, Stream™ Platform, launches globally
Canadian medical technology company, FluidAI Medical (FluidAI), is set to revolutionize postoperative care with the launch of its ground-breaking medical device, the Stream™ Platform in Canada and Saudi Arabia. Through the use of the Stream™ Platform, surgeons have the potential to make a more accurate and timely diagnosis of postoperative leaks, dreaded complications of digestive tract surgeries, which can lead…
Read MoreIotamotion Completes $12m Series A Raise to Accelerate Commercial Expansion of IotaSOFT® Insertion System
iotaMotion, Inc., a leader in developing advanced robotic-assisted systems for cochlear implant surgery, announced today the company has oversubscribed its $12 million Series A capital raise. The company closed the round with support from a select group of venture capital firms, private investors, and current shareholders. The lead investor was Research Corporation Technologies, Inc. (RCT) with participation…
Read MoreUroMems Announces First-Ever Smart Artificial Urinary Sphincter Implant in a Female Patient
UroMems, a global company developing innovative, mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully completed the first-ever implant of the UroActive™ smart, automated artificial urinary sphincter (AUS) in a female patient. This milestone indicates a new era for millions of women suffering from SUI, and results of this clinical study will…
Read MoreFrancis Medical Announces First Patient Treated in VAPOR 2 Pivotal Study for Water Vapor Ablation of Prostate Cancer
Francis Medical, Inc., a privately held medical device company developing an innovative and proprietary water vapor ablation therapy for the treatment of prostate, kidney, and bladder cancer, today announced the first patient has been treated in the company’s VAPOR 2 pivotal clinical study evaluating the safety and efficacy of its Vanquish minimally invasive water vapor…
Read MoreUSMI Receives FDA Approval for New Robotic Surgery Device – The Canady Flex RoboWrist™
US Medical Innovations, LLC (USMI), a Biomedical and Life Science subsidiary of US Patent Innovations, LLC, announced today it has received FDA Approval for its Canady Flex RoboWrist™ to be used in open and laparoscopic procedures in the United States. The device is already approved and used in the Middle East, Europe, and Asia. The…
Read MoreSurmodics Receives FDA 510(k) Clearance for Pounce™ LP Thrombectomy System
Surmodics, Inc. (Nasdaq: SRDX), a leading provider of medical device and in vitro diagnostic technologies, announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Pounce™ LP (Low Profile) Thrombectomy System. Introduced in 2021, the Pounce Thrombectomy System is intended for the non-surgical removal of thrombi and emboli from the peripheral…
Read More