Archive for October 2019
Narbis Launches Smart Glasses Using Neurofeedback and NASA-Developed Algorithm to Encourage Practicing Focus and Concentration
Narbis, a technology company dedicated to developing wellness products that encourage practicing focus and concentration, today announced the online availability of its proprietary Narbis smart glasses that use principles of neurofeedback and an algorithm developed by NASA to discourage distractibility and reward concentration while reading, working on the computer, studying, or doing homework. Narbis smart…
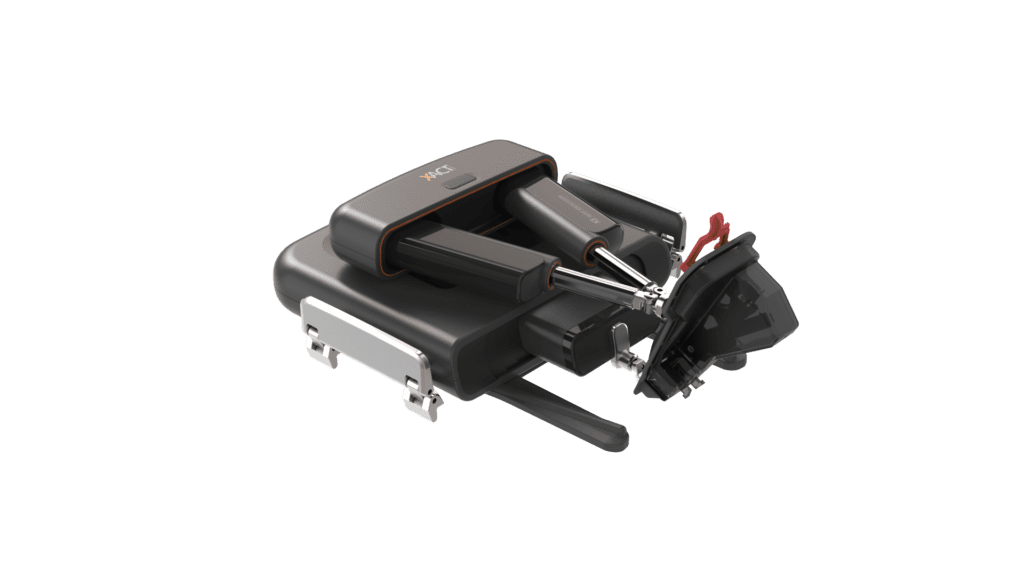
Read MoreFirst-of-its-Kind XACT Robotic System Cleared by FDA for Percutaneous Interventional Procedures
XACT Robotics™ Ltd. today announced that its first robotic system was cleared to market in the U.S. for use during computed tomography (CT) guided percutaneous interventional procedures. XACT’s technology is the first hands-free robotic system combining image-based planning and navigation with insertion and steering of various instruments to a desired target across an array of clinical…
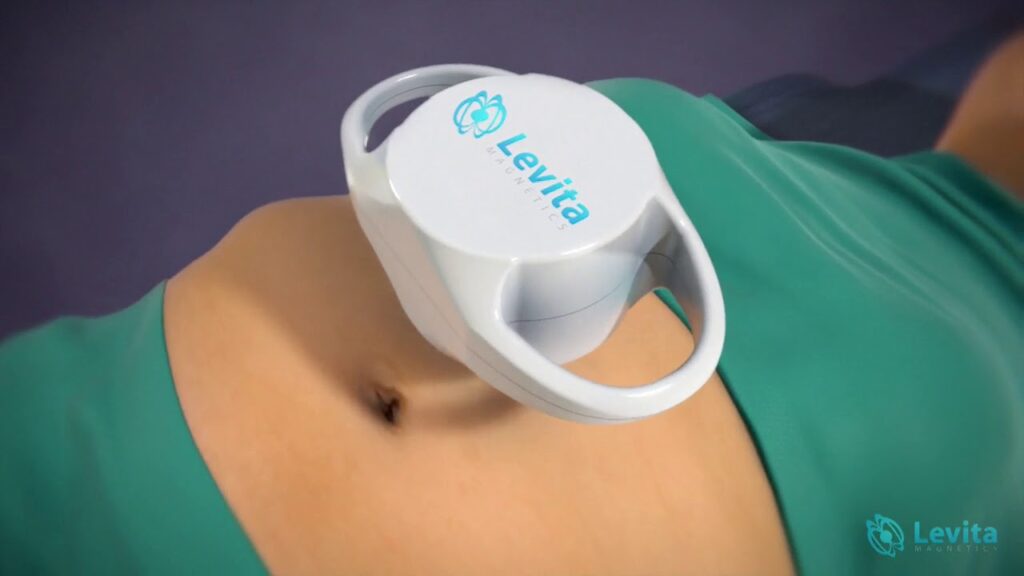
Read MoreLevita Magnetics Announces 1,000th Magnetic Surgery Procedure
Levita Magnetics, a company dedicated to improving surgical outcomes through Magnetic Surgery®, today announced that the 1,000th procedure using Magnetic Surgery has been performed. The platform represents one of the key surgical advancements in the last decade. Indicated to grasp and retract organs in certain surgical procedures, including bariatric surgery, gallbladder removal, and prostate removal,…
Read MoreOnMed Telemedicine Station Connects Patients to Doctors in a Virtual Consultation
OnMed®, an innovative health technology company providing affordable access to quality care, announced a partnership and first deployment of its breakthrough telemedicine station with Tampa General Hospital. Staff members now have access to the OnMed Station for virtual life-sized consultations with physicians and automated pharmaceutical services. The OnMed Station allows users to have real-time consultations…
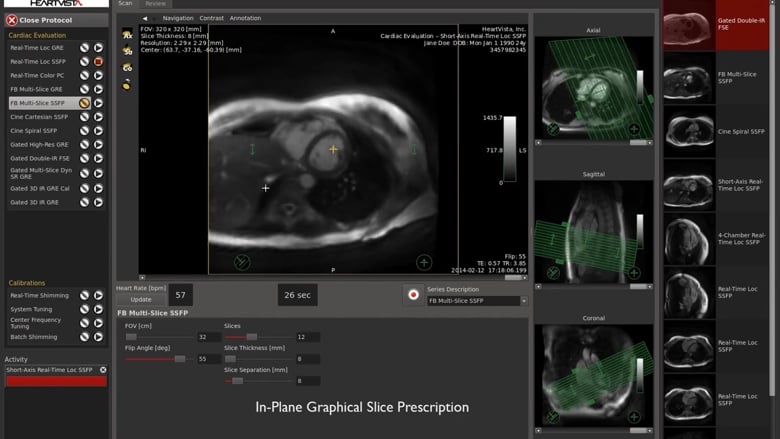
Read MoreHeartVista Receives FDA 510(k) Clearance for One Click™ Cardiac MRI Package, the First AI-assisted Cardiac MRI Scan Solution
HeartVista, a pioneer in AI-assisted MRI solutions, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration to deliver its AI-assisted One Click™ MRI acquisition software for cardiac exams. Despite the many advantages of cardiac MRI, or cardiac magnetic resonance (CMR), its use has been largely limited due to a lack…
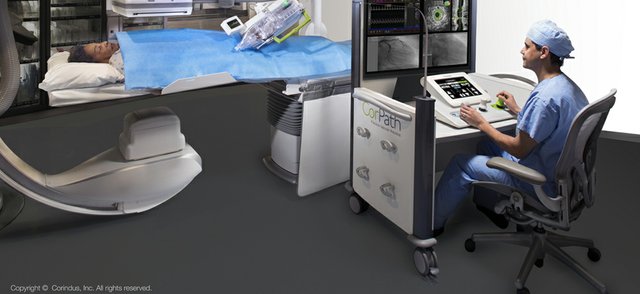
Read MoreSiemens Healthineers Completes Corindus Vascular Robotics Acquisition for $1.1B
Siemens Healthineers AG completed the acquisition of 100 percent of Corindus Vascular Robotics, Inc. effective October 29, 2019. Prior to the closing of the acquisition, Corindus held a shareholders’ meeting on October 25, 2019, at which 87.5 percent of their stockholders approved the acquisition. The relevant governmental authorities previously granted the approvals required to complete…
Read MoreNeuronetics® and Success TMS Partner to Increase Patient Access to Leading Depression Treatment, NeuroStar® Advanced Therapy
Neuronetics, Inc., a commercial stage medical technology company focused on designing, developing and marketing products that improve the quality of life for patients who suffer from psychiatric disorders, today announced a partnership with Success TMS, a healthcare provider specializing in transcranial magnetic stimulation (TMS), a non-drug, non-invasive treatment for adult patients with Major Depressive Disorder…
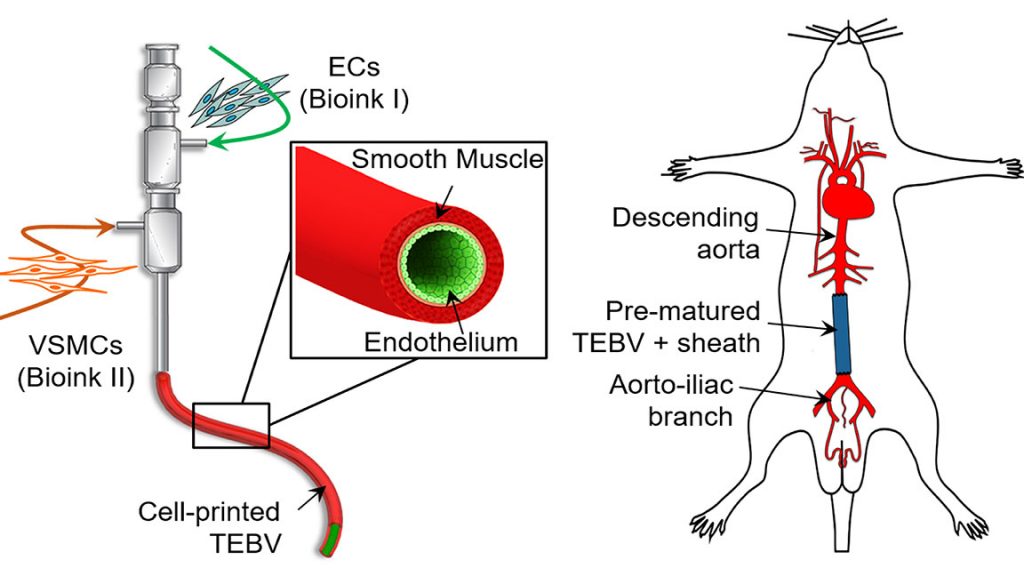
Read More3D Printed Cells and Bioinks for Making Implantable Blood Vessels
A biomimetic blood vessel was fabricated using a modified 3D cell printing technique and bioinks, which were formulated from smooth muscle cells from a human aorta and endothelial cells from an umbilical vein. The result is a fully functional blood vessel with a dual-layer architecture that outperforms existing engineered tissue and brings 3D-printed blood vessels…
Read MoreU.S. FDA Clears GI Scientific’s ScopeSeal®, the Only Single-Use Disposable Device Indicated to Significantly Reduce Duodenoscope Contamination
GI Scientific, LLC, a developer of transformative innovations for gastrointestinal disease, announced that the U.S. Food and Drug Administration (FDA) cleared its ScopeSeal® Duodenoscope Protective Device, the first Endoscopic Shield® for protecting the distal end of a duodenoscope from contamination during ERCP procedures. ScopeSeal® is a single-use disposable infection control device that preserves duodenoscope optics and other…
Read MoreB9 Creations Unveils Their New, Cutting-Edge 3D Printer
B9Creations, a global provider of 3D printing solutions, announced today the launch of its new additive manufacturing Healthcare Division & Service Bureau and new medical upgrade to its award-winning B9 Core Series 3D printer line with the launch of the B9 Core Med 500 and the first two of its suite of biocompatible materials. With…
Read More