Archive for November 2022
Desert Imaging Deploys densitas® intelliMammo™ A.I. Solutions for Breast Screening Services
Densitas® Inc., a global provider of A.I. solutions for digital mammography and breast screening announced that Desert Imaging has implemented the densitas® IntelliMammo™ platform across their health system. The adoption of intelliMammo™ reinforces Desert Imaging’s established commitment to maintaining robust organization-wide quality improvement initiatives. IntelliMammo™ provides Desert Imaging with on-demand actionable information and streamlined workflows to establish…
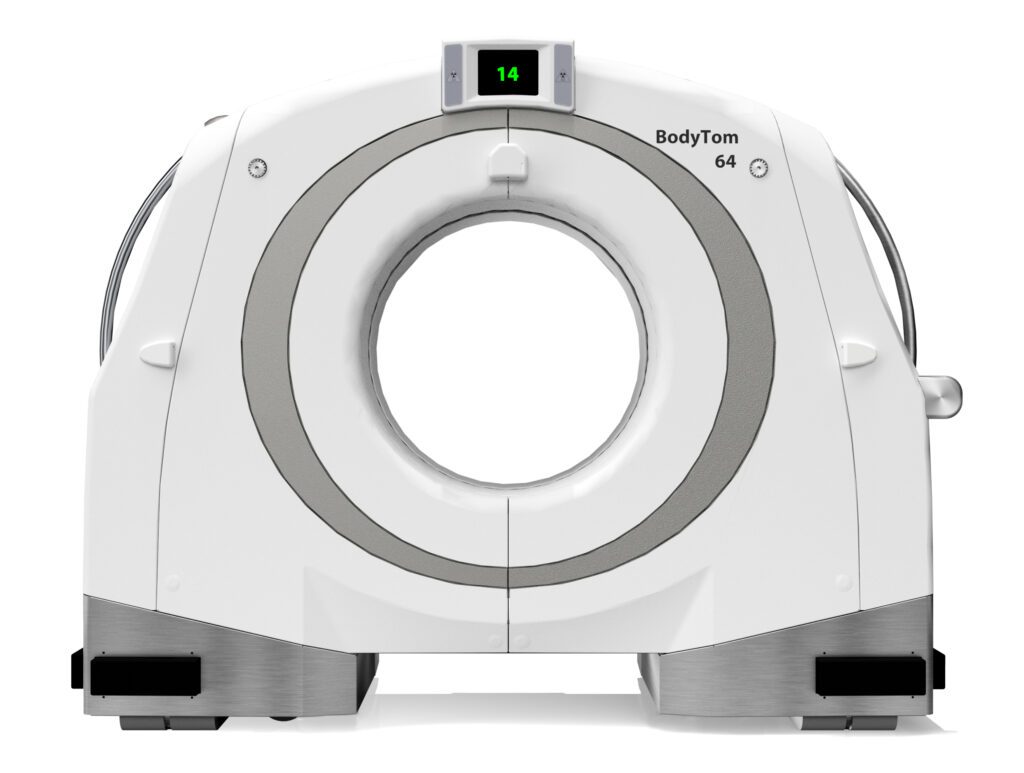
Read MoreNeuroLogica Announces FDA 510(k) Clearance of BodyTom® 64
NeuroLogica Corp., a subsidiary of Samsung Electronics Co. Ltd., announced that its head-to-toe trauma imaging solution, the BodyTom® 64 Point-of-Care Mobile Computed Tomography (CT) Scanner, has received 510(k) clearance from the U.S. Food and Drug Administration for commercial use in the United States. “We’re thrilled to build off our expertise and elevate point-of-care imaging with…
Read MoreWhat Are You Thankful For? | Legacy MEDSearch 2022
The holiday season always brings a time of family, friends, good food, and also reflection on the year. This year as we are in the season of giving thanks, we asked our team what everyone was thankful for this holiday season. Here is what they had to say! “I’m thankful for my family; my wife…
Read MoreChoiceSpine™ Announces Line Extension of their Harrier™ Stand-Alone Anterior Lumbar Interbody Fusion System
ChoiceSpine LLC, a privately-held spinal device manufacturer based in Knoxville, TN, announces the launch of an additional lordotic offering for their stand-alone anterior lumbar interbody – Harrier SA. Harrier SA is a 3D printed stand-alone screw-based system incorporating ChoiceSpine’s proprietary BioBond™ porous structure technology. The system has had great success since launching in May of…
Read MoreNew View Surgical, Inc. Closes $12.1M Series B-1 Financing, Readies Limited Market Release of its VisionPort™ System For Minimally Invasive Surgery
New View Surgical, Inc., an emerging medical device company developing proprietary imaging and access technologies for minimally invasive surgery (MIS), announced the closure of a $12.1M Series B-1 equity financing round to fund the commercialization of its VisionPort™ System. The company expects to launch the VisionPort System in the US before the end of 2022.…
Read MoreElemental Machines Raises $41M in Series B Funding to Fuel Growth
Elemental Machines, a developer of the LabOps Intelligence Platform for labs around the world, has raised $41 million in a Series B funding round led by Sageview Capital and co-led by Omega Venture Partners, with continued participation from Gutbrain Ventures and Digitalis Ventures. “The support we have received from Sageview Capital, Omega Venture Partners, and our existing…
Read MoreFDA Clearance Awarded to Tyber Medical for NiTy+ One-Shot Staple Fixation System
Tyber Medical LLC, the global, leading private-label medical device provider for major orthopedic trauma, extremity, and spine companies, has been awarded a U.S. Food and Drug Administration (FDA) 510(k) clearance for its new NiTy+ One-Shot™ Staple Fixation System. The NiTy+ One-Shot is the first instrument of its kind in the bone-fixation device market to feature a…
Read MoreXironetic Receives FDA Clearance for Augmented Reality Surgical Software
Xironetic, an early-stage healthcare technology company, today announced its IntraOpVSP™ augmented reality (AR) visualization software for complex surgeries has received clearance from the U.S. Food and Drug Administration (FDA). IntraOpVSP displays patient CT and MRI as three-dimensional holograms in AR headsets, helping surgeons visualize surgical plans, anatomical targets, and cutting guides alongside or overlayed on…
Read MoreReCor Medical Announces Consistent Reduction of Blood Pressure in Pooled Analysis of Three Clinical Trials at AHA 2022
ReCor Medical, Inc. (“ReCor”) and its parent company, Otsuka Medical Devices Co., Ltd. announced consistent and significant blood pressure (“BP”) lowering results across a range of patients with uncontrolled hypertension, including across differences in age, sex, baseline blood pressure, medication level and ethnicity. The results come from analysis of the pooled data from ReCor’s RADIANCE…
Read MoreSaranas Announces Over 1,200 Patients Treated with the Early Bird® Bleed Monitoring System
Saranas, Inc. announced that over 1,200 patients have been treated with the Early Bird® Bleed Monitoring System, the first and only FDA-approved bleed detection system. The Early Bird was launched in 2019 following a De Novo classification by the U.S. Food and Drug Administration. The device monitors and detects endovascular bleed complications through a novel…
Read More