Cardiovascular / Cardiology
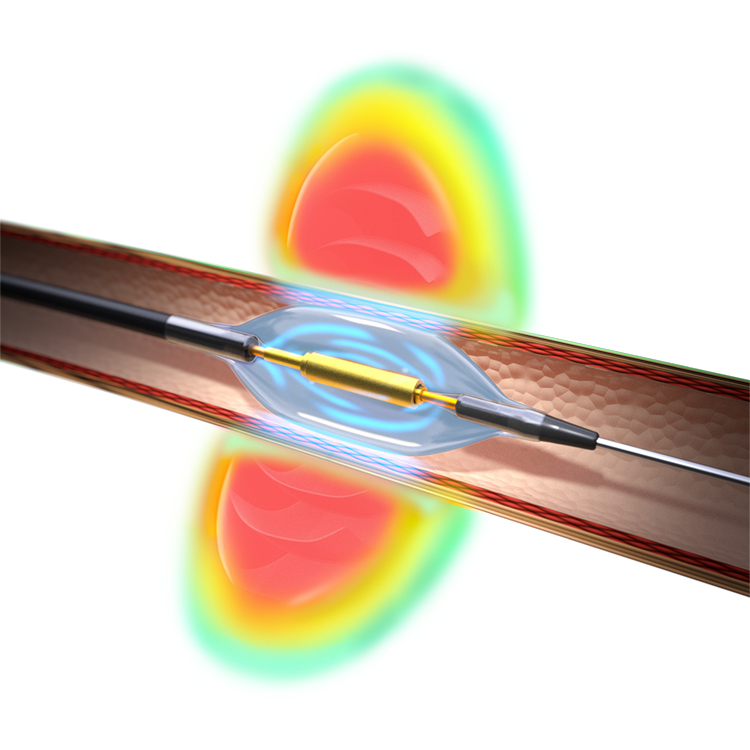
FDA greenlights Boston Scientific’s Novel Drug-Coated Balloon for Coronary In-Stent Restenosis
Boston Scientific’s AGENT™ Drug-Coated Balloon (DCB) has been granted approval by the U.S. Food and Drug Administration (FDA) for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. While advancements in percutaneous coronary intervention (PCI) technologies are ongoing, recent research indicates that ISR, the gradual re-narrowing of a previously opened coronary artery, still…
Read MoreSparrow BioAcoustics Launches Software That Turns a Smartphone into a Stethoscope
Sparrow BioAcoustics recently unveiled its StethophoneTM software, which transforms smartphones into medical-grade stethoscopes. This innovative tool enables users to capture, analyze, and share crucial heart health data with medical professionals from any location. In June 2023, Sparrow received FDA clearance for the downloadable application, making it the first FDA-cleared smartphone stethoscope software-as-a-medical-device (SaMD). The company…
Read MoreFDA Grants ExThera Multiple Breakthrough Device Designations for its Seraph® 100 Filter
ExThera Medical Corporation, a healthcare company developing and manufacturing extracorporeal pathogen technologies, announces that it has received multiple Breakthrough Device Designations from the U.S. Food and Drug Administration (FDA), for its Seraph® 100 Microbind® Affinity Blood Filter (Seraph 100). The Seraph 100 is a patented blood filter containing a biomimetic surface, similar to that found…
Read MoreQT Medical® Announces Completion of Series B Financing of $12 Million
QT Medical, Inc., a medtech company focused on cardiac care technology, announced today that it raised $12 million in Series B financing. This funding will accelerate QT Medical’s market penetration, global expansion, product pipeline development, and long-term strategic planning. QT Medical aims to provide improved cardiac devices and services to revolutionize the care of patients with heart disease.…
Read MoreInspira™ Announces 510(k) FDA Submission of INSPIRA™ ART100 Towards Commercialization
Inspira™ Technologies OXY BHN Ltd. (Nasdaq: IINN, IINNW) (the “Company” or “Inspira Technologies”), a company aiming to bring a paradigm shift to acute respiratory care by empowering breathing without lungs, announced it had submitted its INSPIRA ART 100, a cardio-pulmonary bypass device, to the U.S. Food and Drug Administration (FDA) via the 510(k) pathway, with potential clearance…
Read MoreFDA Approves LimFlow System in Patients With Chronic Limb-Threatening Ischemia and No Suitable Endovascular or Surgical Revascularization Options
LimFlow SA, a pioneer in the development of minimally-invasive technology for the treatment of chronic limb-threatening ischemia (CLTI), a severe form of peripheral artery disease (PAD), announced today that the U.S. Food and Drug Administration (FDA) has approved the LimFlow System to help people with CLTI who have no other suitable endovascular or surgical treatment…
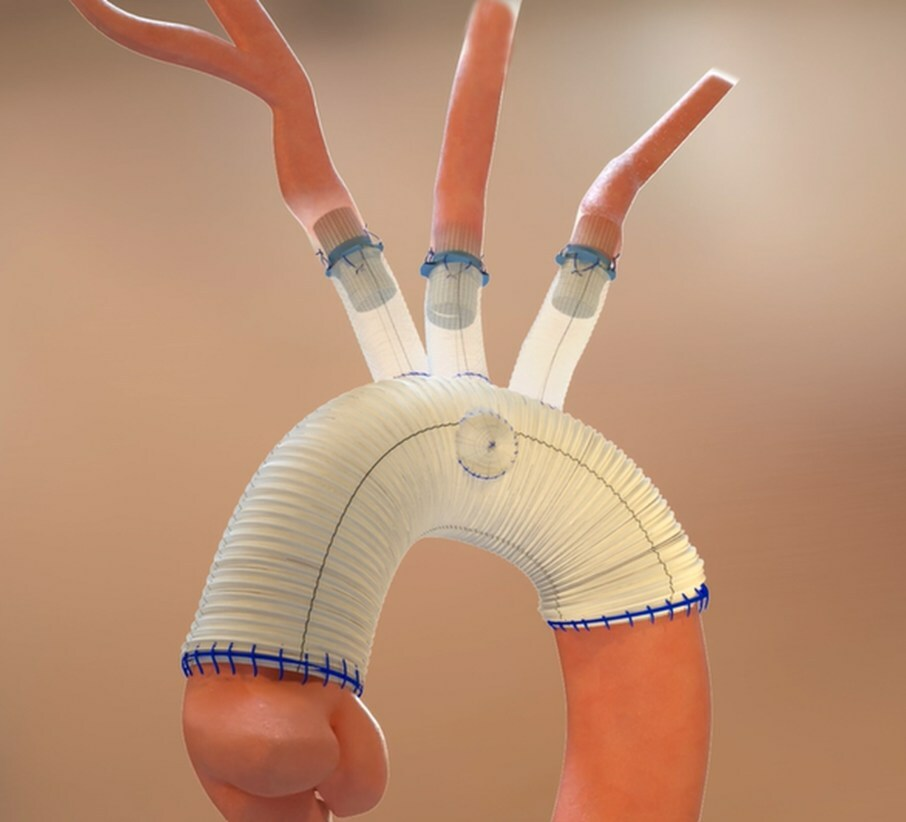
Read MoreAquedeon Medical, Inc. Receives FDA IDE Approval for the Duett Vascular Graft System
Aquedeon Medical, Inc., a Silicon Valley pioneering medical device company specializing in novel cardiothoracic solutions, is pleased to announce a significant milestone following receipt of FDA Investigational Device Exemption (IDE) approval to conduct a staged pivotal clinical trial for its Duett Vascular Graft System in the United States. The study will be initiated in the second…
Read MoreRecor Medical and Otsuka Medical Devices Announce Positive Vote from the FDA Advisory Committee Meeting on the Paradise™ Ultrasound Renal Denervation System
Recor Medical, Inc. (“Recor”) and its parent company, Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”) announce the U.S. Food and Drug Administration (FDA) Circulatory Systems Devices Panel of the Medical Devices Advisory Committee met to discuss the pre-market approval application (PMA) for the Paradise™ Ultrasound Renal Denervation (RDN) system, indicated to reduce blood pressure in…
Read MoreAcorai receives Breakthrough Device Designation for their Non-Invasive Intracardiac Pressure Monitor
Acorai, a start-up medical device manufacturer from Sweden, today announces that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for Acorai’s Heart Monitor, a device for the non-invasive estimation of diastolic pulmonary artery pressure (dPAP), systolic pulmonary artery pressure (sPAP), and mean pulmonary artery pressure (mPAP) in patients with Stage C Heart…
Read MoreCapstan Medical Leverages Robotics to Bring Minimally Invasive Care to Heart Valve Patients
Capstan Medical, a developer of minimally invasive technology to address heart valve disease, today announced an oversubscribed $31.4 million Series B investment led by Eclipse. Additional participating investors include Intuitive Ventures and Puma Venture Capital, a new firm founded by Amit Hazan, a veteran Wall Street medical technology analyst, and Dr. Vipul Patel, a renowned robotic surgeon who…
Read More