Archive for February 2022
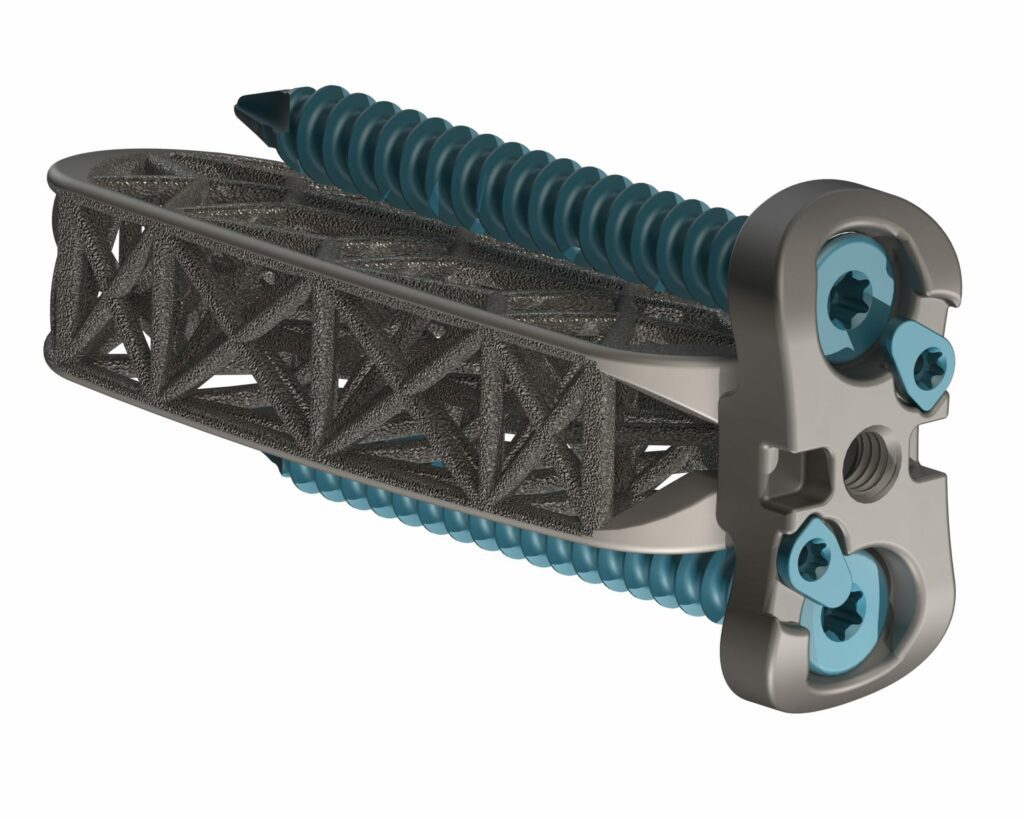
4WEB Medical Launches Hyperlordotic Lateral Implant Portfolio
4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design utilizing its proprietary Truss Implant Technology™, announced the company’s launch of a comprehensive array of hyperlordotic lateral implants. The first procedure was performed by Brad Prybis, MD, a surgeon at Tanner Medical Center in Carollton, GA. “The application of 4WEB’s…
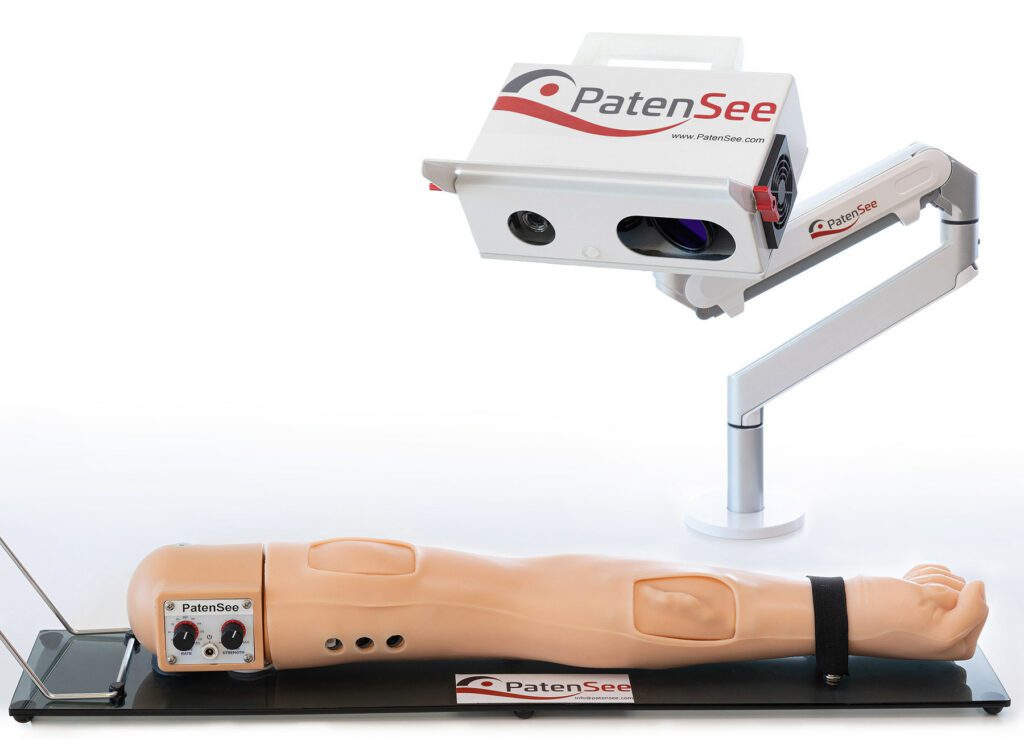
Read MorePatenSee’s Contactless Imaging Device Shows Promise in Monitoring Dialysis Patients
The interim results of an ongoing clinical study of PatenSee‘s contactless imaging device for the early detection of vascular access stenosis for dialysis patients were shared in a poster presentation. The presentation, titled First Clinical Experience with Non-Invasive, Contactless, Optical Surveillance of Vascular Access, was delivered by the study’s principal investigator, Professor Benaya Rozen-Zvi, MD at the 2022 World…
Read MoreEvoEndo Announces US FDA 510(k) Clearance for their Single-Use, Unsedated Transnasal Endoscopy System
EvoEndo®, Inc. (“EvoEndo”), a medical device company developing systems for Unsedated Transnasal Endoscopy (TNE), has announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA) to begin marketing and sale of the EvoEndo® Single-Use Endoscopy System. The clearance follows EvoEndo’s distribution agreement with Micro-Tech Endoscopy USA, Inc. (“Micro-Tech”), which will begin…
Read MoreRapid Medical Granted FDA Breakthrough Device Designation for Vasospasm Treatment
Rapid Medical’s Comaneci is the first device that allows physicians to monitor vessel expansion, apply incremental adjustments, and enhance treatment with combination therapeutics. With this unprecedented control, the FDA designated Comaneci as a breakthrough device–offering advantages over existing technology to improve safety and efficacy in cerebral vasospasm. Rapid Medical, a leading developer of advanced neurovascular…
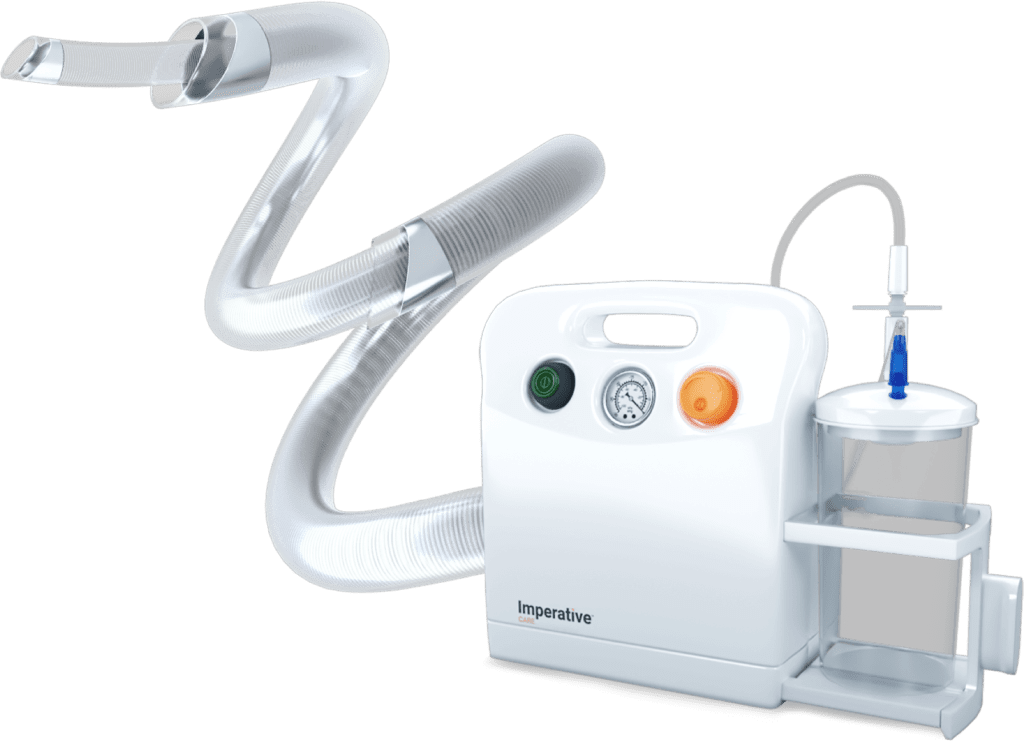
Read MoreImperative Care Launches Its Zoom POD™ for Stroke Treatment, the First and Only Filter to Capture Clot in the Sterile Field
Imperative Care, Inc. announced the launch of its Zoom POD™ Aspiration Tubing, the company’s latest innovation in elevating stroke care. The Zoom POD is the newest addition to Imperative Care’s Zoom Stroke Solution™, the company’s ischemic stroke product portfolio that includes the Zoom 88 Large Distal Platform for neurovascular access, four Zoom Aspiration Catheters in various…
Read MoreFirst Hospital on the East Coast to Perform Total Knee Replacement with THINK Surgical’s Next-Generation Robot System
Hackensack University Medical Center, a national leader in orthopedic care and robotic surgery, recently completed the first total knee replacement on the East Coast using the newest generation of the TSolution One robot from THINK Surgical. The system, manufactured by the Fremont, CA – based company features a true, active robot which supports a choice of knee implants…
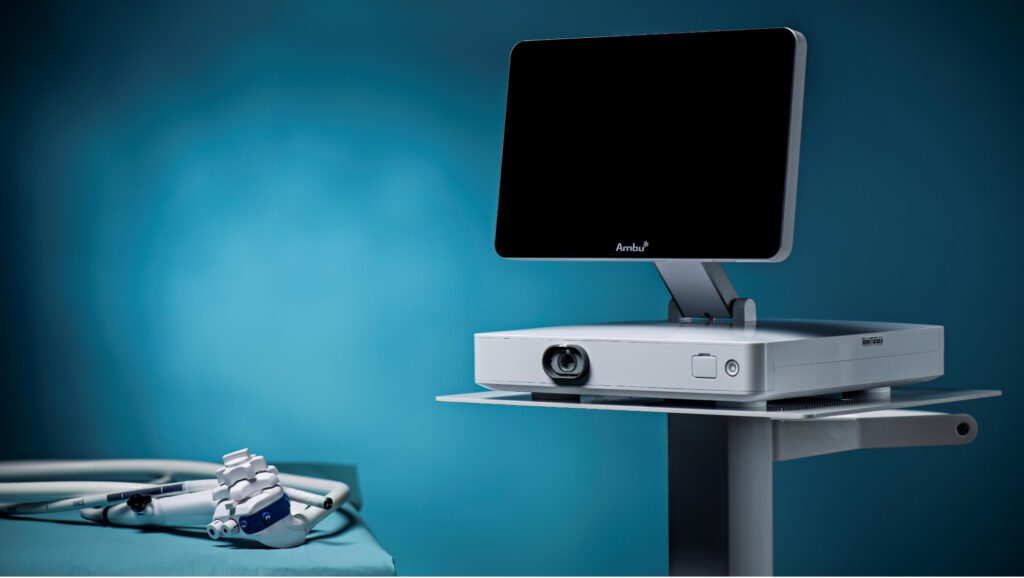
Read MoreAmbu Announces FDA Clearance of Single-Use Gastroscope and Next-Generation Display Unit
Ambu announces the 510(k) regulatory clearance of the Ambu® aScope™ Gastro and Ambu® aBox™ 2 in the United States. aScope Gastro is Ambu’s first sterile single-use gastroscope and includes new advanced imaging and design features in a combined solution with next-generation display and processor technology. With HD capabilities, the aBox 2 will set a new…
Read MoreA New Suturing Tool, Endomina System, from Endo Tools Therapeutics, Gains FDA Clearance
Endo Tools Therapeutics (ETT), developers of advanced endoscopic medical devices for use by gastroenterologists, today announces the U.S. Food and Drug Administration (FDA) 510(k) clearance of the endomina® system, designed for endoscopic placement of suture(s) and approximation of soft tissue in the gastrointestinal tract on adult population. The endomina system is comprised of a universal…
Read More