Archive for January 2023
Axonics Receives FDA Approval for Fourth-Generation Rechargeable Sacral Neuromodulation System
Axonics, Inc., a global medical technology company that is developing and commercializing novel products for the treatment of bladder and bowel dysfunction, announced that the U.S. Food and Drug Administration has approved the company’s fourth-generation rechargeable sacral neuromodulation system. The newly approved Axonics R20™ neurostimulator is labeled for a functional life in the body of at…
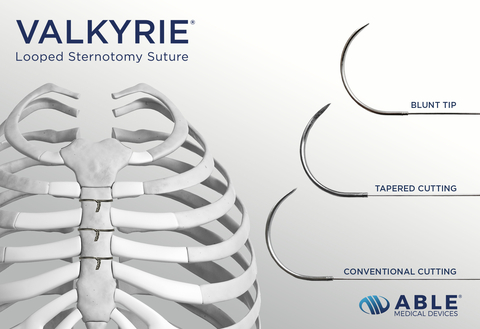
Read MoreAble Medical Announces Launch of Valkyrie® Looped Sternotomy Sutures
Able Medical Devices announced the launch of Valkyrie® Looped Sternotomy Sutures. The stainless-steel looped wire sutures are used to close a patient’s chest after open heart surgery. When compared to traditional wire sutures, the Valkyrie Looped Suture doubles the surface area of single wires and provides a more robust sternal closure. Valkyrie Looped Sternotomy Sutures…
Read MoreThe Legacy MEDSearch Layoff Survival Guide
As the economy slows, layoffs, unfortunately, become more common. It’s never easy to be laid off, but our team has a couple of tips to share to help you recover quickly and find a new role that is right for you. It can be hard to stay calm in an overwhelming and unexpected situation,…
Read MoreConventus Flower Orthopedics Announces Expansion of its Flex-Thread™ Technology Platform with FDA Clearance of the Ulna Intramedullary Nail System
Conventus Flower Orthopedics, an innovation-driven medical technology company, announced they have received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Flex-Thread™ Ulna Intramedullary (IM) Nail System. The Flex-Thread Ulna Nail marks a new application and portfolio expansion to the company’s Flex-Thread technology platform. Expected to launch in February 2023, the Flex-Thread Ulna IM Nail…
Read MoreFDA Clears Cytovale’s® IntelliSep® Sepsis Test, First in a New Class of Emergency Department-Focused Diagnostic Tools
Cytovale®, a medical diagnostics company focused on advancing early detection technologies to diagnose fast-moving and immune-mediated diseases, announced today that its IntelliSep® test has received U.S. Food and Drug Administration (FDA) 510(k) clearance to aid in the early detection of sepsis for the approximately 30 million adult patients with signs and symptoms of infection who present…
Read MoreHip Innovation Technology Announces Initiation of FDA Approved Investigational Device Exemption Study
Hip Innovation Technology, LLC (HIT), a medical device company developing innovative orthopaedic device solutions to advance the quality of life and quality of care for patients, is pleased to announce the first U.S. implantation of the HIT Reverse Hip Replacement System (Reverse HRS), under an FDA approved Investigational Device Exemption (IDE). The IDE Study is…
Read MoreMedcura Receives Breakthrough Device Designation for its LifeGel™ Absorbable Surgical Hemostat
Medcura, Inc., a medical device company dedicated to transformatively improving the management of surgical bleeding, announces that the U.S. Food and Drug Administration (FDA) has granted the coveted Breakthrough Device Designation for its LifeGel™ Absorbable Surgical Hemostat. LifeGel™ is the first and only hemostatic agent to receive Breakthrough Device Designation seeking a new and highly…
Read MoreMicronoma Receives FDA Breakthrough Device Designation for OncobiotaLUNG, A Novel Liquid Biopsy Assay for Lung Carcinoma Detection
Micronoma, the first biotech company offering early cancer detection with a microbiome-driven liquid biopsy platform, announced that its OncobiotaLUNG assay received the Breakthrough Device Designation from the Food and Drug Administration (FDA). As a result, Micronoma can expect continued guidance and prioritized reviews from the agency of its upcoming clinical trial and concomitant pre-market approval…
Read MoreVERO Biotech Receives FDA Approval of its Third Generation Tankless Inhaled Nitric Oxide Delivery System
VERO Biotech Inc., a commercial-stage healthcare business dedicated to neonatal intensive care and the acute care hospital community, announced FDA approval of the newest generation of its tankless inhaled nitric oxide (iNO) delivery system. The Third Generation GENOSYL® Delivery System – developed for respiratory therapists by respiratory therapists – has new features that are expected to deliver three key benefits…
Read MoreLydus Medical Announces FDA Clearance of Vesseal™, The Microvascular Anastomosis Aid Device for Small Arteries
Lydus Medical is pleased to announce that the Vesseal™ has received FDA clearance 510(k). The Vesseal™ is a microvascular anastomosis suture deployment system, for standardized omni-vessel anastomoses, enabling simple, fast, safe, and effective procedures. An anastomosis is one of the most complicated steps of microvascular surgery and fundamental to the success of these demanding surgical interventions. Varied clinical…
Read More