Archive for January 2023
ABM Respiratory Care Announces the FDA Clearance of the Biwaze Clear System
ABM Respiratory Care, a medical technology company focused on developing and globally commercializing novel integrated airway clearance and ventilation solutions, announced the U.S. Food and Drug Administration (FDA) 510(k) clearance of the BiWaze® Clear System. This new airway clearance system helps patients clear their airways as well as prevent or treat atelectasis by using Oscillating Lung…
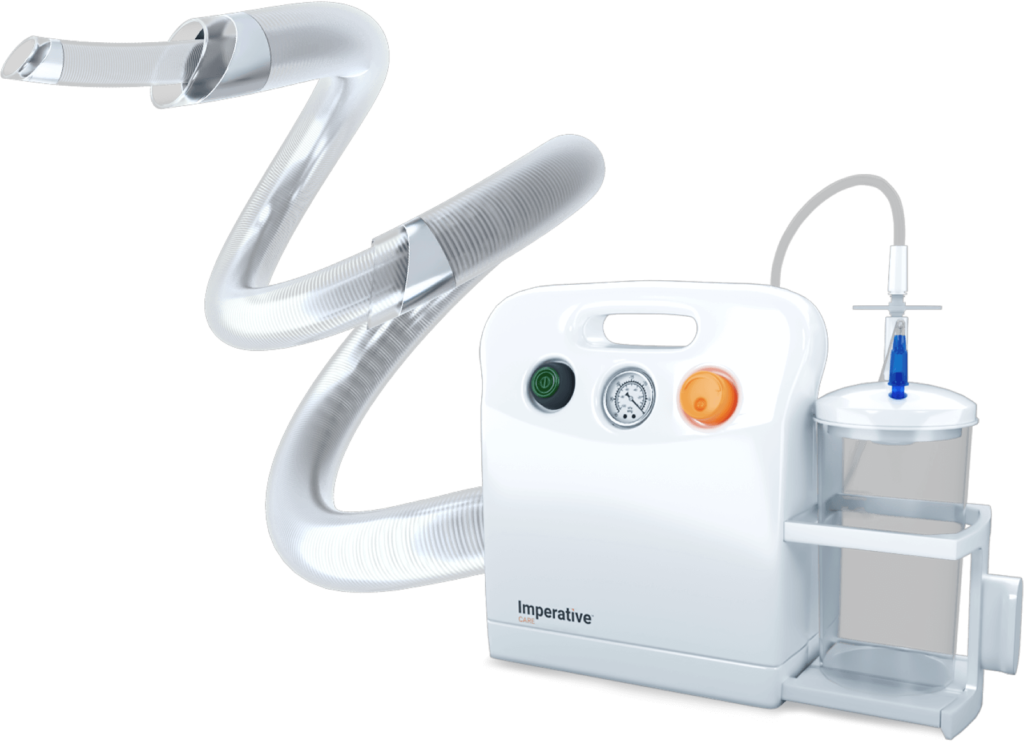
Read MoreImperative Care Announces FDA Clearance and Initial Cases of Zoom RDL, the First Stroke-Specific Radial Access Platform for Mechanical Thrombectomy
Imperative Care, Inc., announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Zoom™ RDL Radial Access System, the company’s latest innovation in elevating stroke care and the first radial access platform developed specifically for ischemic stroke treatment. Zoom RDL is the newest addition to Imperative Care’s Zoom Stroke Solution™, the company’s ischemic stroke…
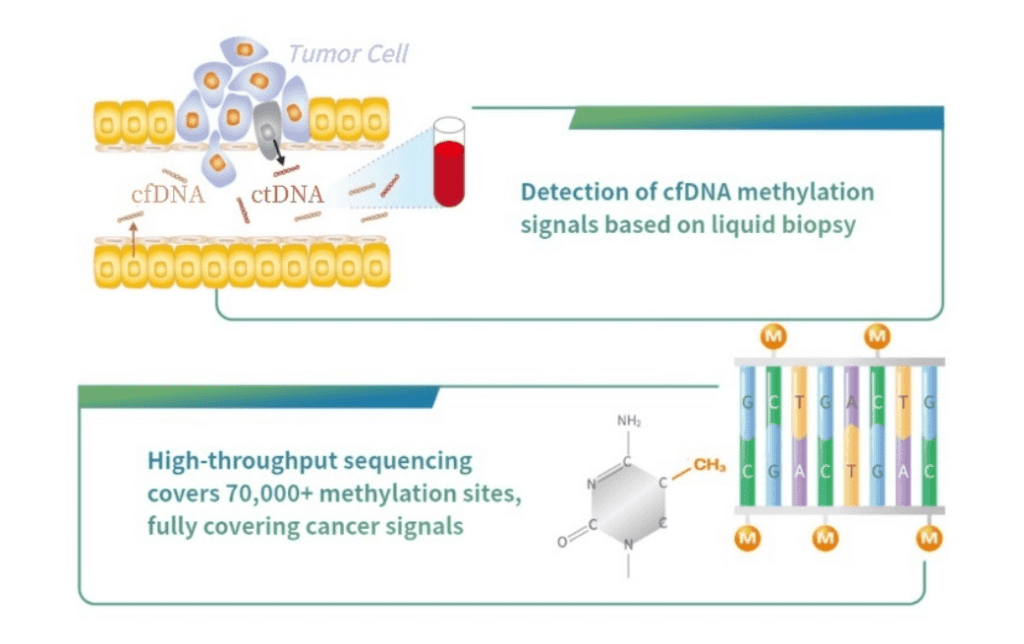
Read MoreBurning Rock Received FDA Breakthrough Device Designation for its OverC™ Multi-Cancer Detection Blood Test
Burning Rock, a company focused on the application of next generation sequencing (NGS) technology in the field of precision oncology, announced that its OverC™ Multi-Cancer Detection Blood Test (MCDBT) has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA), which is the third of its kind globally. Under the FDA’s Breakthrough…
Read MoreVisby Medical™ Receives FDA Emergency Use Authorization for Respiratory Health Test for use in CLIA waived settings
Visby Medical™ announced that it has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration for its Respiratory Health point of care test. The Visby Medical Respiratory Health Test is a fast, polymerase chain reaction (PCR) device that detects and differentiates between upper respiratory infections caused by Influenza (Flu) A & B, and SARS-CoV-2 (COVID-19). Visby Medical has…
Read More